Bexion Media
Press Release
Bexion Pharmaceuticals, Inc. toPresent at BIO InternationalConvention 2024
COVINGTON, Ky., May 31, 2024 /PRNewswire/ -- Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company developing a new generation of biologic therapy to treat solid tumor cancers and chemotherapy-induced peripheral neuropathy (CIPN), today announced that the Company will present at the 2024 Biotechnology Innovation Organization (BIO) International Convention taking place June 3-6, 2024, in San Diego, California. Presentation Details: Location: Theater 1, Hall A, Exhibition Hall Session Date and Time: Wednesday, June [...]
Cincinnati Enquirer: 8 Cincinnati biotech companies with big steps to watch in 2020
Excerpt: Cincinnati has become a nurturing place for bright ideas in biotechnology, and 2020 could bring big advancements. The Cincinnati USA Regional Chamber of Commerce estimates the industry contributes more than $3 billion to the area economy. Nearly 800 locations employ more than 13,000 workers who are making average salaries of about $100,000 a year. In alphabetical order, here are eight Cincinnati biotechs aiming for milestones in 2020. Bexion Pharmaceuticals [...]
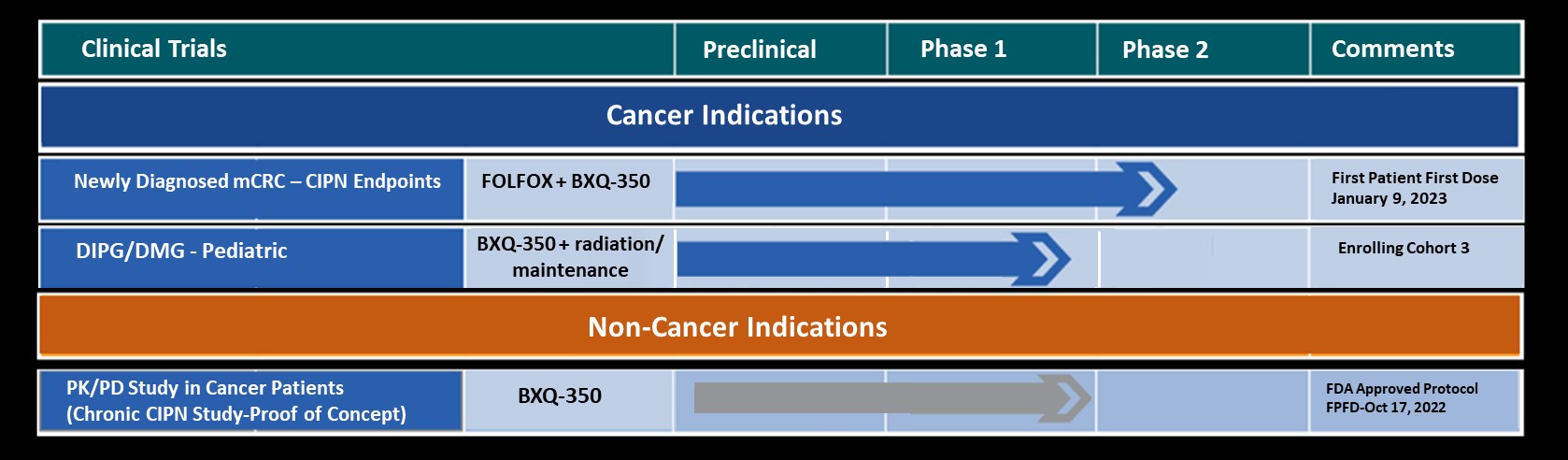
Abstracts
Posters
Case Studies
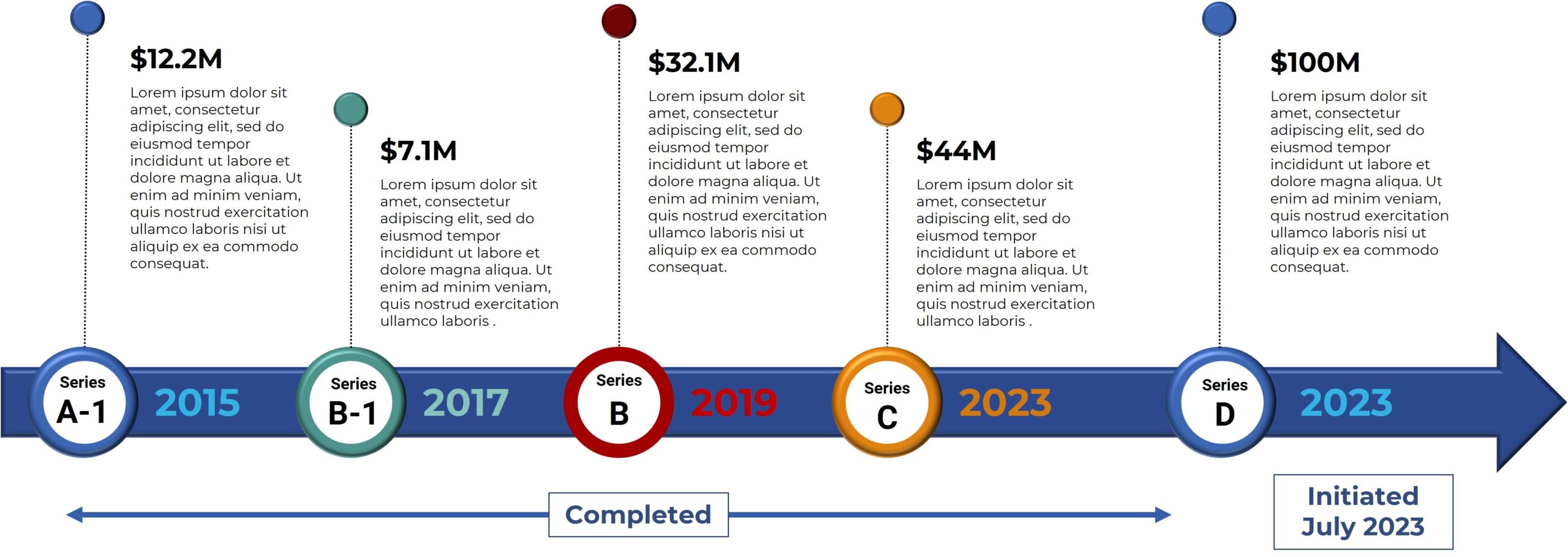
Phase 1 Patient 1080-001:
Long Lasting Benefit in mCRC >5 years
PET/CT before BXQ-350
PET/CT after 18 months on BXQ-350
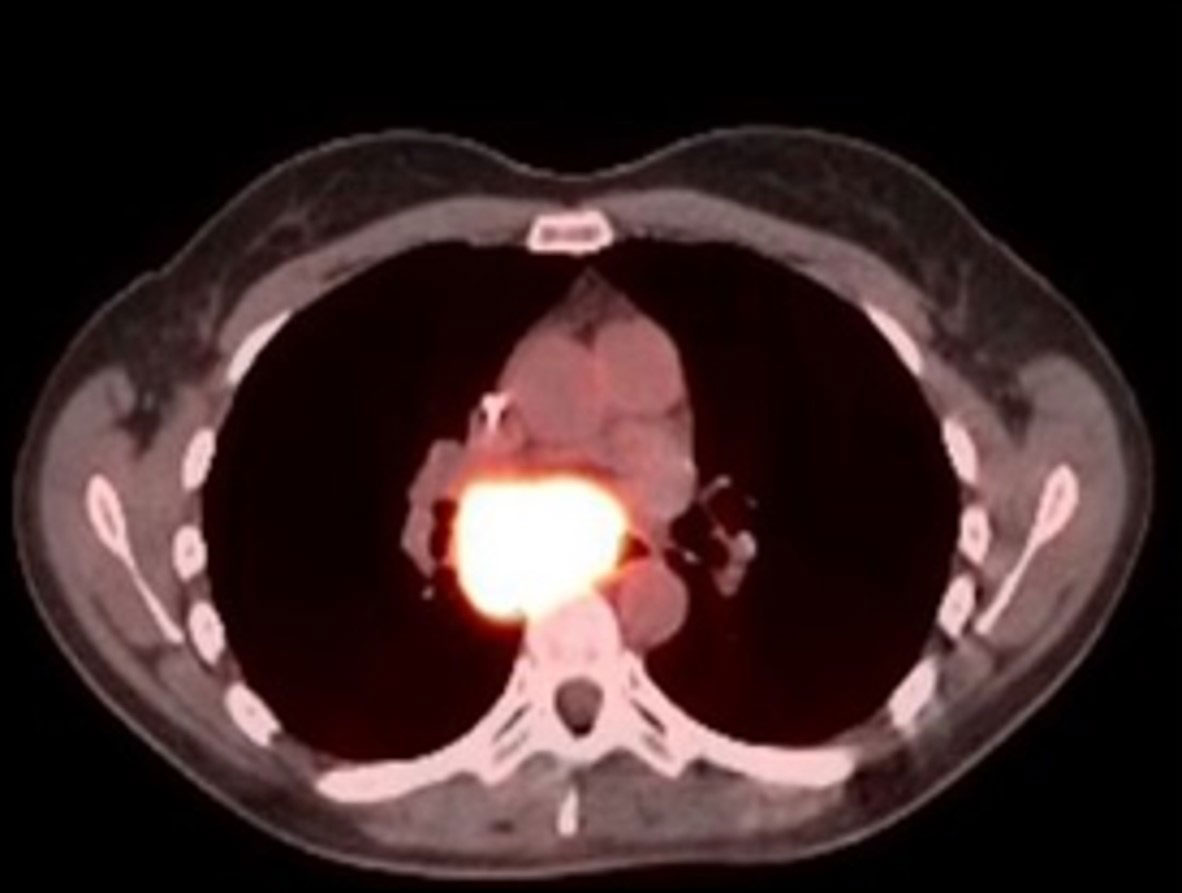
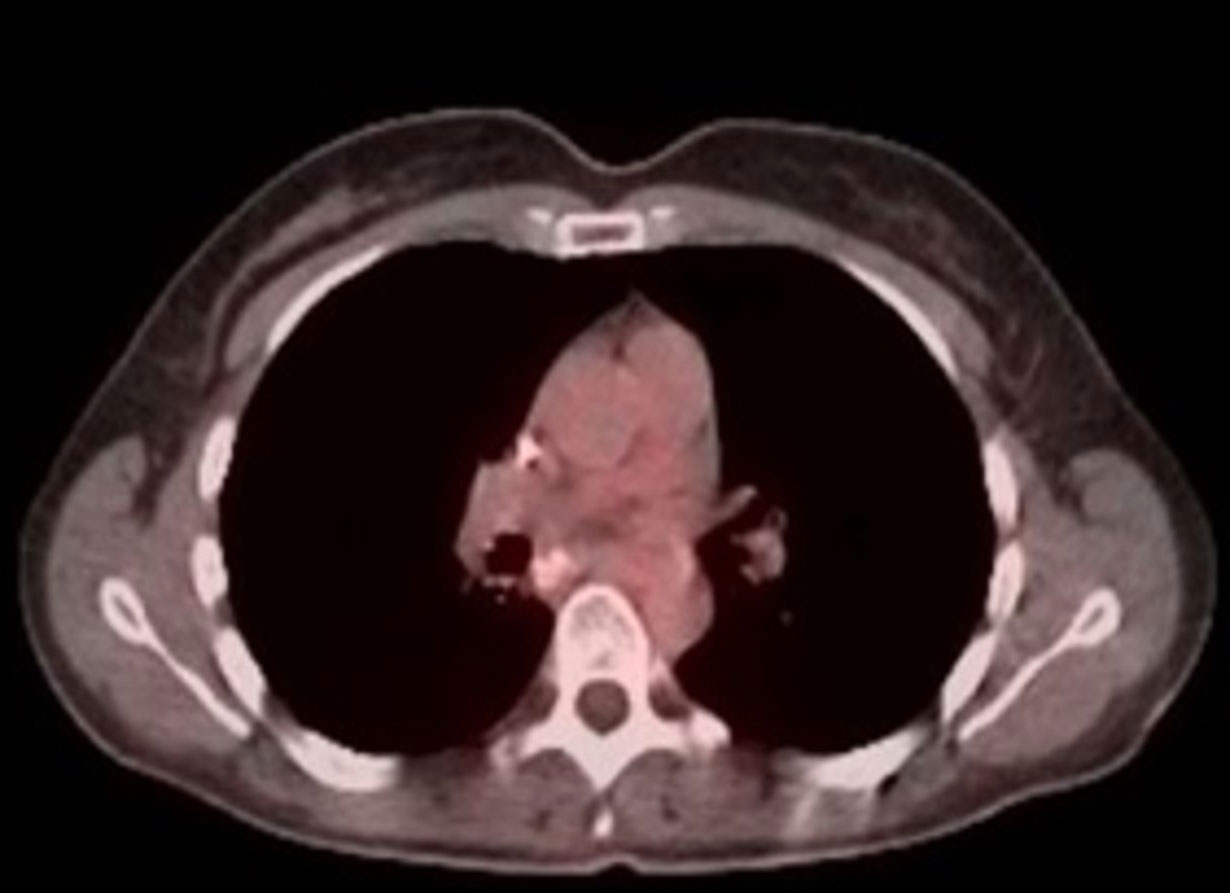
- 40-yr old female with stage 4 metastatic colorectal carcinoma
- Diagnosed in Nov 2015, previously treated with surgery, chemotherapy and radiation (>3 lines)
- Rapid progression (5 months) prior to starting BXQ-350
- Target lesion (1.5 cm) remained unchanged per Recist
- Still Stable Disease over 5 years on study
Phase 1 Patient 1008-701:
Long Lasting Benefit in GBM > 5 years!
Screening
Day 56
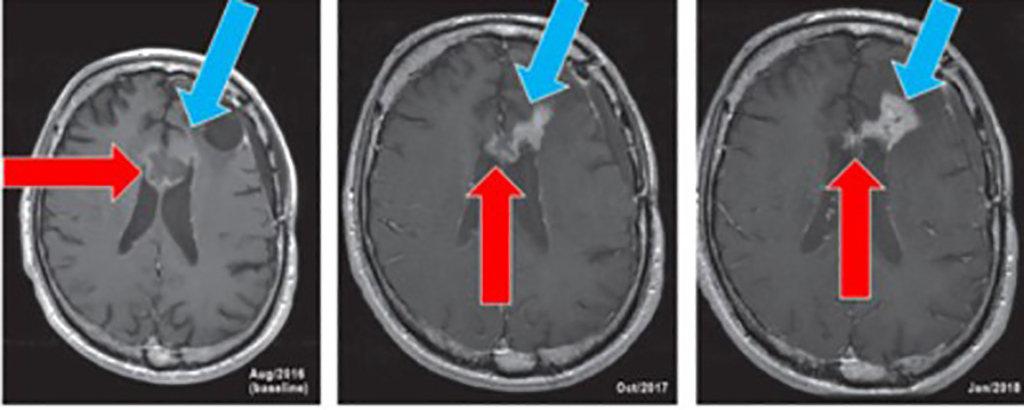
- Red Arrows indicate the initial target lesion that decreased in size while on study
- Blue Arrows indicate new area of enhancement
- Note: Surgery in May, 2018 revealed significant treatment effect with only trace tumor cells present. As of September, 2019 patient continues on study having completed 3 years in the trial.
Case Data: Glioblastoma (GBM)
- Axial T1-post contrast (Ax SE T1 POST FC) 1.5T MRI of the brain of a 64-yr old male patient with glioblastoma (GBM) treated with BXQ-350. GBM was first diagnosed in 2013 and previously treated with surgery, chemotherapy and radiation. Measurable lesion reduction over time is apparent in the anterior corpus callosum and frontal lobe, with those bordering the ventricle.
Phase 1 Patient 1075-213:
Partial Response in Glioblastoma (-74%)
Screening
Day 56
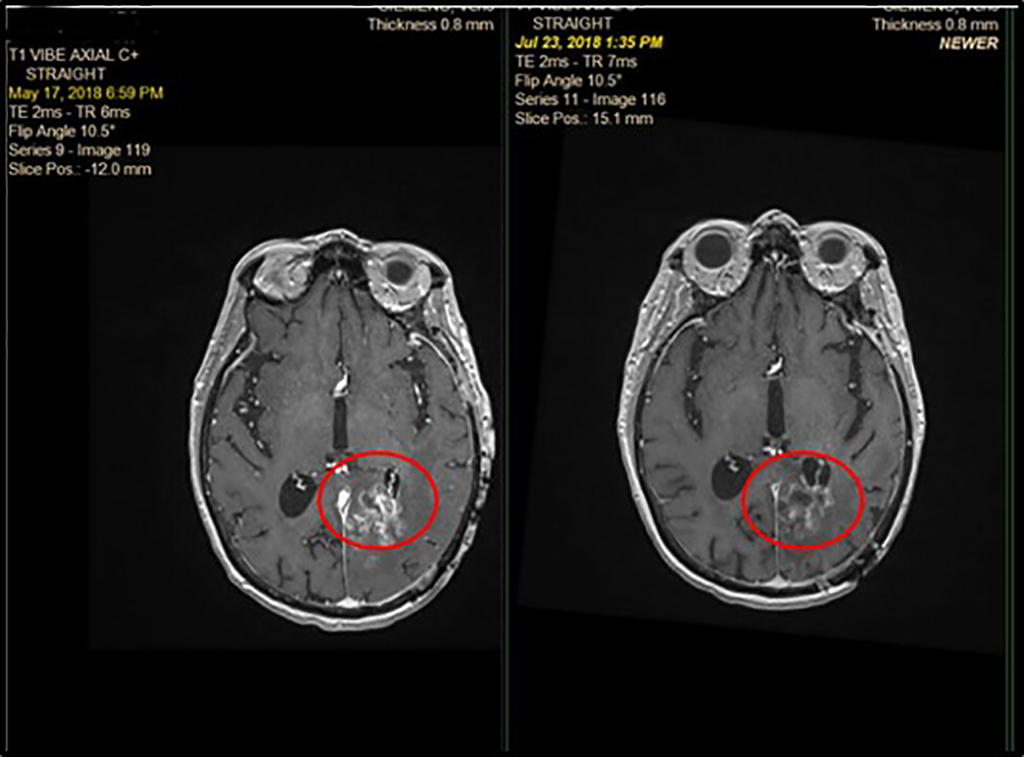
- 65-yr old female with rGBM
- GBM diagnosed in March 2017 (stage IV) previously treated with surgery, chemotherapy and radiation
- Rapid progression (2M) prior to starting BXQ-350 in May 2018
- 1 target lesion (L parietal) 1.4 cm at Screening down to 0.36 cm at Day 56 (-74%)
- Progressed after 948 days on BXQ-350