Clinical Trials
Before a new therapy can be made available to the general public, multiple studies involving patient volunteers must be conducted to evaluate the safety and effectiveness of the therapy. These studies, referred to as clinical trials, are regulated by the Food and Drug Administration (FDA) in the United States and by similar regulatory agencies worldwide.
Initial clinical safety results have been promising. A robust clinical development plan is being initiated to maximize the potential of our BXQ-350 platform.
Information about Bexion’s current clinical trials can be found below:
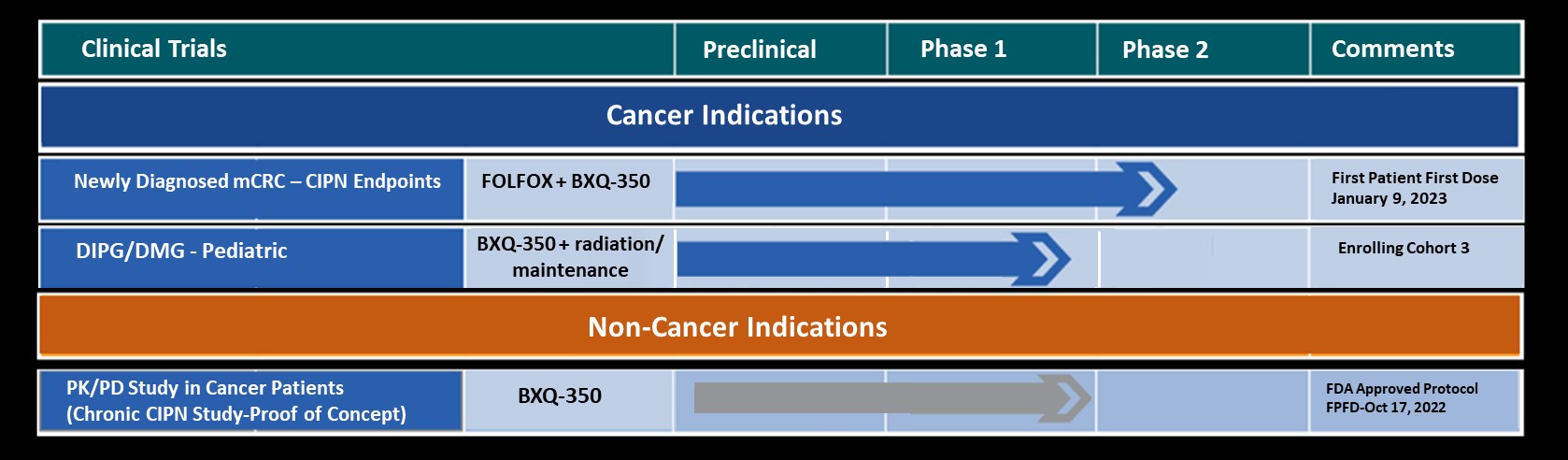
A S I S T S T U D Y (open)
Phase 1b/2 Placebo Controlled, Double Blinded Study on the EfficAcy and Safety of BXQ-350 In Combination with mFOLFOX7 and Bevacizumab in Newly DiagnoSed MeTastatic Colorectal Carcinoma
Trial can be found at https://clinicaltrials.gov/ct2/show/NCT05322590
R E T R O S T U D Y (Chronic CIPN Study-Proof of Concept) (open)
Pilot PRoof of ConcEpT PhaRmacOkinetic/Pharmacodynamic (PK/PD) Study in Cancer Patients
Trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT05291286
E T E R N I T I S T U D Y (enrolling by invitation)
ContinuEd TrEatment foR ParticipaNts Enrolled In STudIes of BXQ-350
Trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT04404569
K O N Q U E R S T U D Y (open)
A PharmacoKinetic and Safety EvaluatiON Study of BXQ-350 in Children With Newly Diagnosed DiffUsE IntRinsic Pontine Glioma (DIPG) or Diffuse Midline Glioma (DMG)
Trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT04404569
K O M P A S S S T U D Y (closed)
A pharmacoKinetic and safety study of BXQ-350 in cOMPlex Advanced Solid tumors and high-grade gliomaS in adult patients.
Trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT02859857
K O U R A G E S T U D Y (closed)
A Phase 1 pharmacoKinetic and safety Study of BXQ-350 in children and yoUng adults with RelApsed solid tumors, includinG rEcurrent malignant brain tumors.
Trial can be found at https://www.clinicaltrials.gov/ct2/show/NCT03967093
Bexion has completed enrollment in Phase 1 studies in both adults and children with advanced solid tumors utilizing BXQ-350 as monotherapy. The results showed evidence of anti-tumor activity and also demonstrated that BXQ-350 has a tolerable safety profile with no dose limiting toxicity (DLT) at the highest administered dose.
More information about clinical trials:
Access to Investigational Medicines
Investigational medicines are medicines that are being tested to determine if they are safe and effective. They have not been approved by regulatory authorities (such as the Food and Drug Administration) for their specific use. They may or may not be effective in the treatment of the intended condition and may cause unexpected side effects. Patients with serious diseases or conditions, or those who have exhausted all other treatment options may benefit from investigational treatments. When seeking access to an investigational medicine, it is critical that patients and their physicians consider all possible risks and benefits.
Clinical Trials
Clinical trials provide the safest opportunity for patients to access investigational medicine before it is approved by a regulatory agency. By volunteering to take part in a clinical trial, participants receive closely monitored care from health care providers managing the trial and help others by contributing to medical research.
Clinical trials allow Bexion Pharmaceuticals, Inc. to evaluate the safety and effectiveness of its investigational new treatments. These data are necessary to obtain regulatory approval of those treatments and then make them available to the broader patient population.
To participate in a trial, patients must meet certain criteria and provide written informed consent acknowledging their willingness to accept the potential risks and benefits of the investigational treatment. Information about Bexion’s clinical trials can be found at www.bexionpharma.com/clinical-trials or www.clinicaltrials.gov.
Expanded Access
Expanded access (sometimes referred to as compassionate use) is a potential pathway for patients with a serious or immediately life-threatening disease or condition to gain access to an investigational medicine outside of a clinical trial when no other treatment options are available.
The FDA has prepared guidelines related to expanded access, including a “question and answers” guideline (FDA guideline titled: Expanded Access to Investigational Drugs for Treatment Use —Questions and Answers, Guidance for Industry, June 2016, Updated October 2017).
Access to Bexion’s investigational products outside of a clinical trial would be considered only under limited circumstances, and as permitted by applicable law, in the following situations:
In addition, Bexion will consider granting expanded access to an investigational medicine only if all the following criteria are also met:
Bexion encourages patients to first speak to their treating physician about clinical trial eligibility and enrollment options. If the physician believes that expanded access may be the only option, the physician should contact Bexion to request information about applying for access on behalf of the patient. This will enable the physician to work directly with Bexion to determine the best course of action. It is important to note that the patient’s physician must oversee all aspects relating to the potential use of the drug under expanded access (e.g., regulatory compliance, IRB approval, patient treatment, adverse event management, and reporting).
Physicians may contact Bexion at 1-800-746-3915 or by email at [email protected]. Bexion will typically acknowledge receipt of a request for expanded access within 3-5 business days.
Bexion will carefully review all expanded access requests in consideration of the above criteria. If approved by Bexion, final approval of the expanded access by the regulatory authority in the country where the request originated is required before shipment of any investigational medicine will occur. All physicians who receive investigational medicine from Bexion through expanded access are required to comply with all applicable laws and regulations, and contractual conditions, including those relating to safety reporting.
More information can be found at https://www.fda.gov/news-events/public-health-focus/expanded-access