Bexion Pharmaceuticals Announces Leadership Additions
The new leadership roles support the company’s momentum in becoming the leader in developing and commercializing therapeutics based on exploiting the lysosome to treat challenging solid tumors as well as chemotherapy-induced peripheral neuropathy (CIPN).
FOR IMMEDIATE RELEASE [Covington, KY—February 2, 2022]
Bexion Pharmaceuticals, a clinical stage biopharmaceutical company, today announced key leadership changes, including the appointment of Richard “Scott” Shively as the Company’s President and Chief Executive Officer, and member of the board of directors. Mr. Shively will succeed Dr. Ray Takigiku, the Company’s Founder and current Chief Executive Officer and President. Dr. Takigiku will continue with the Company as Founder and Chief Scientific Officer, leading all scientific, clinical, research and development efforts, and advising company strategy.
“I want to thank Ray for his pioneering vision and exceptional leadership over the past 15 years,” said Chuck Scheper, Chairman of Bexion’s board. “Ray has taken an innovative drug compound and developed a thriving biopharma organization, whose products will improve human health.”
“Founding Bexion Pharmaceuticals and providing the guidance to grow this organization has been a focal point of my career”, commented Dr. Takigiku. “Scott is a very experienced pharma leader with a history of growing successful businesses. As I continue to provide scientific and development guidance to Bexion, I am confident Scott is the right person to succeed in achieving the next level of financial growth to ensure Bexion’s commercial success.”
“I am delighted to be joining Bexion at this exciting time for the company and thrilled to lead it through the next stages of growth”, said Shively. “Ray Takigiku, the team, and the Board have done an excellent job progressing cutting edge cell biology science through early-stage development. The company’s lead product, BXQ-350, is now poised for phase 2 development in solid tumor cancers such as colorectal and challenging forms of pediatric glioblastoma, as well as CIPN (Chemotherapy Induced Peripheral Neuropathy). I look forward to working with this outstanding team.”
Mr. Shively is an experienced Founder, CEO and CCO in the US and international biopharma markets. He previously served as CEO and Co-founder of Neumentum, Inc., a private clinical development stage company focused in the Pain and CNS therapeutic areas. Over the course of his career, Mr. Shively has achieved outstanding financial results and growth in value for shareholders by developing innovative strategies, acquiring assets, building highly talented teams, raising capital, and successfully launching new products, and has been involved in several successful exits for mid-sized biopharma companies. In addition to his C-suite experience with several small rapid growth companies, Shively spent a number of years at Pfizer where he led global commercial efforts for the Pain and CNS areas, including responsibility for products such as LYRICA® (pregabalin) and CELEBREX® (celecoxib) as well as the development pipeline.
About Bexion Pharmaceuticals
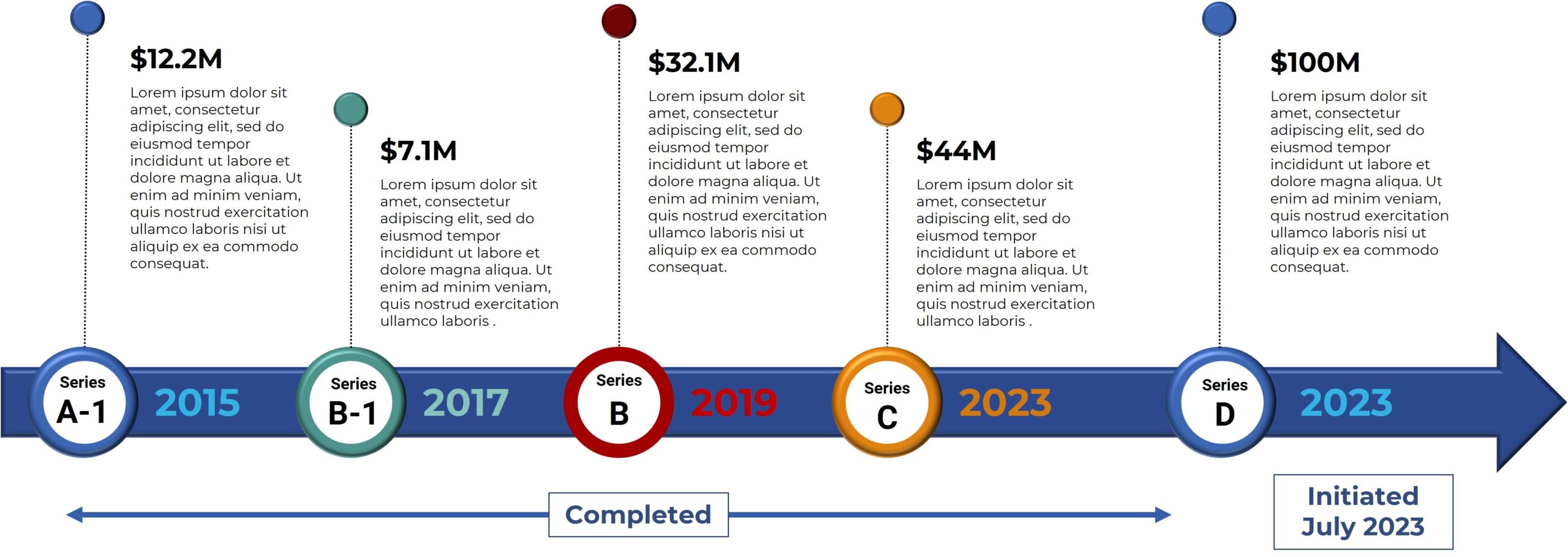
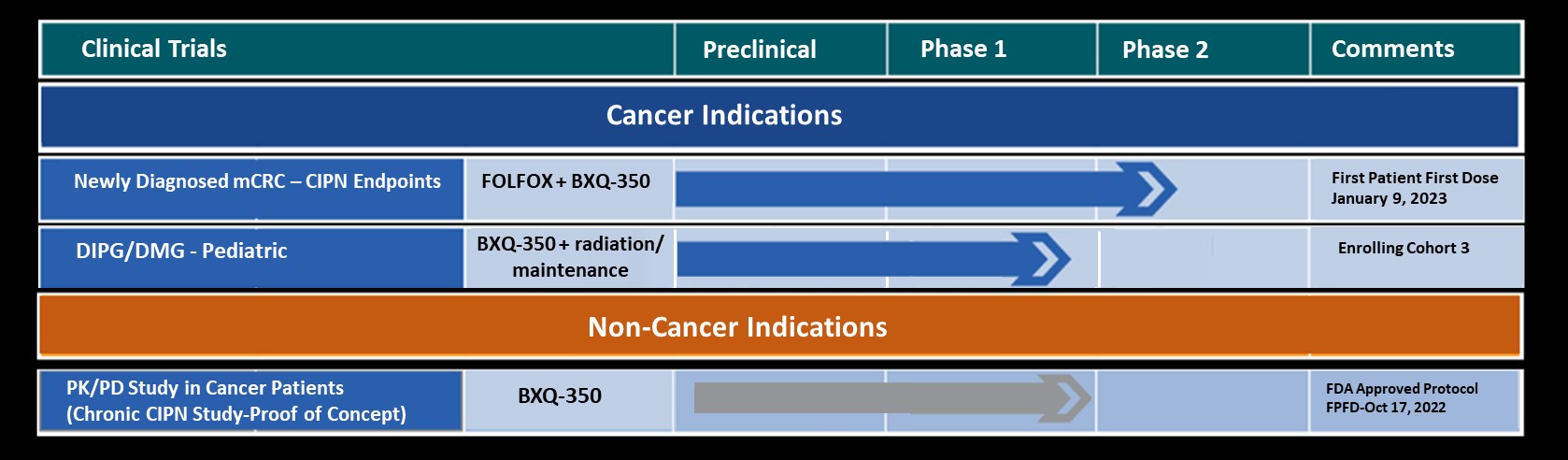
Bexion Pharmaceuticals, a clinical-stage biopharmaceutical company, is pioneering the development of life-changing treatments by leveraging the untapped mechanisms of the lysosome. Bexion believes the lysosome is an underexploited cellular orchestrator involved in multiple diseases. Bexion’s lead drug candidate is BXQ-350, a first-in-class biologic containing the multifunctional, lysosomal activator protein, Saposin C and a phosphatidylserine. BXQ-350 has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in brain and other solid tumors, including those that may lead to brain metastases. Bexion has completed a multisite first-in-human Phase 1 clinical trial of BXQ-350 for solid tumors and gliomas. Bexion is in Phase 1/2 for a rare pediatric brain tumor and plans to initiate two adult Phase 2 programs in 2022.
Media Contact:
Margaret van Gilse ● 859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward-looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.