Bexion Pharmaceuticals, Inc. Announces Data at 2018 ASCO Annual Meeting from Ongoing Phase I BXQ-350 Clinical Trial
FOR IMMEDIATE RELEASE Covington, KY ~ June 12, 2018
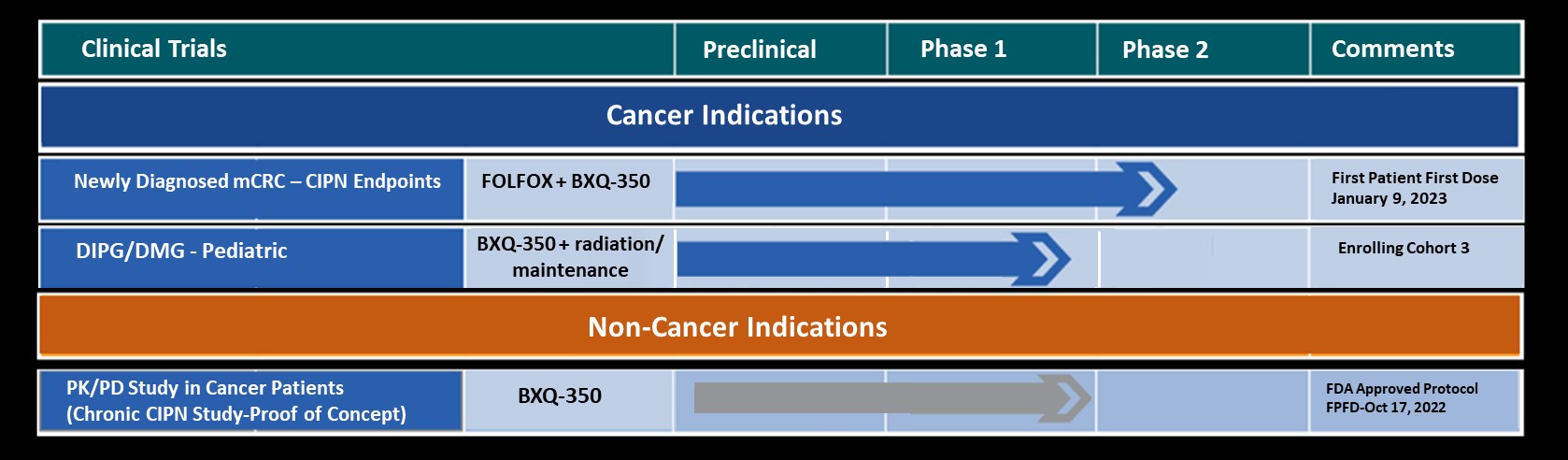
Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company focused on rare brain and solid tumors, discussed data from 17 patients enrolled in the Phase Ia portion of its ongoing Phase I Safety Trial at the American Society of Clinical Oncology (ASCO) Annual Meeting Poster Session, held in Chicago June 1-5, 2018.
In the poster presentation entitled, “First-in-class Phase Ia Study of BXQ-350 for Solid Tumors and Gliomas”, the preliminary data showed:
- 9 patients with Glioblastoma Multiforme (GBM); 8 patients with other solid tumors
- Patients had a median 7 prior systemic therapies
- No Dose Limiting Toxicities (DLTs)
- No treatment –related serious adverse events (SAEs)
- Most common treatment-related moderate AEs were transient fatigue
- Best response in 7 patients completing to day 113:
- 1 Partial Response (appendiceal carcinoma)
- 6 Stable Disease (improved day 113 RANO/RECIST
- 1 High Grade Glioma Stable Disease >19+ months
“Bexion’s team was excited to share our Phase Ia data at the ASCO conference,” stated Dr. Ray Takigiku, Founder and CEO. “With this promising data indicating the potential for a tumor agnostic approach, Bexion is now enrolling patients with solid tumors and gliomas in Phase 1b, and we are initiating efforts to towards a Phase 1 trial in pediatrics and combination Phase 2 studies in adults”.
About BXQ-350
BXQ-350 is a unique formulation of a synthetically produced, human lysosomal protein, Saposin C (sphingolipid activator protein, or SapC), and the phospholipid dioleoylphosphatidylserine (DOPS).
About Bexion Pharmaceuticals
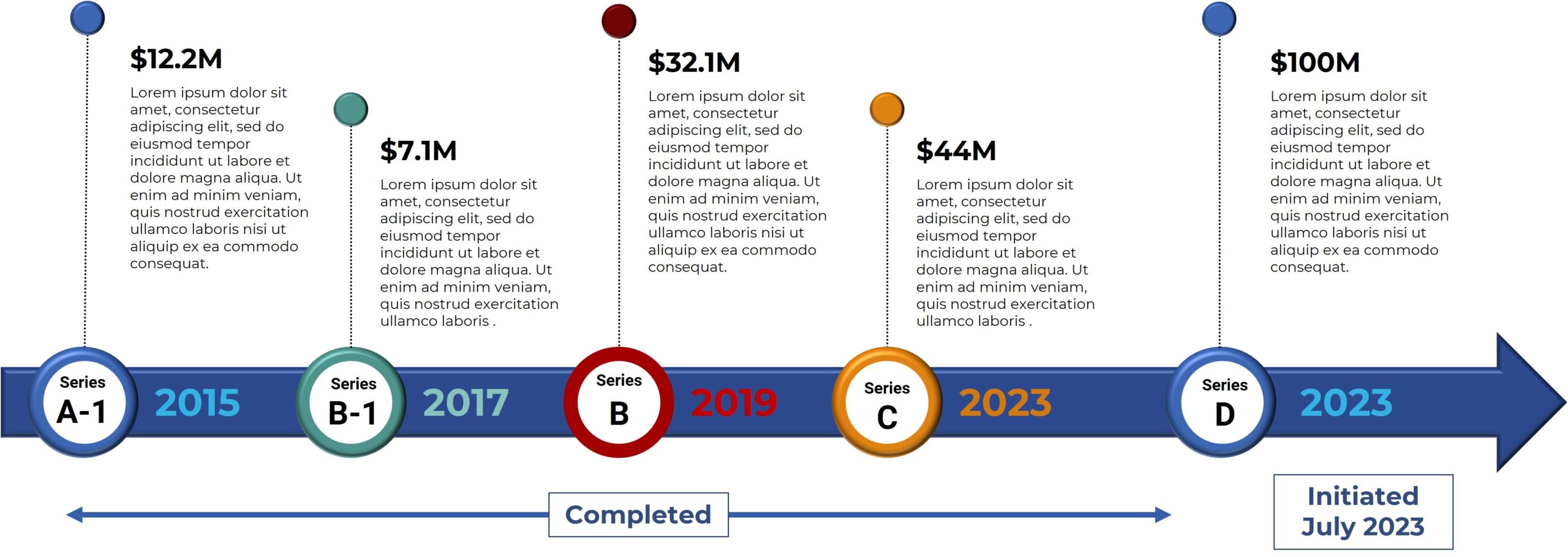
Bexion Pharmaceuticals is a privately-held biotech company focused on the development and commercialization of innovative cures for cancer. Bexion’s first-in-class biologic, BXQ-350, has demonstrated selective tumor targeting with the potential for clinical efficacy in a broad range of cancers. In 2013 the NCI awarded Bexion a prestigious “Bridge Award” of $3MM to support testing of BXQ-350 in the clinic. In February 2015, the FDA granted Bexion Orphan Drug status for Saposin C, the active ingredient in its proprietary drug BXQ-350, for the potential treatment of glioblastoma multiforme (GBM), a type of brain cancer. In June 2015, Bexion won a Tibbett’s Award by the Small Business Administration for exemplifying the very best in innovation. Currently Bexion is in a Series B Fund Raise. For more information, visit bexionpharma.dev.neptuneweb.com
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.