Changing the Narrative
Bexion is changing the narrative on the fight against cancer with BXQ-350, our lead compound. BXQ-350’s novel mechanisms of action impact the tumor and its microenvironment and modulate Ceramide and S1P, bioactive sphingolipids that play a critical role in stemming cancer and pain.

BXQ-350: Multiple Mechanisms of Action
S1P Reduction Properties
S1P promotes the proliferation of cancer cells, activates oncogenic pathways, and stimulates immuno-suppressor cells.
Analysis of preclinical and clinical samples in Phase 1 clinical trials demonstrated that BXQ-350 increases ceramides and decreases sphingosine-1-phosphate (S1P).
Modulate Tumor microenvironment
Saposin C, a component of BXQ-350 has a strong affinity for phosphatidylserine.
Phosphatidylserine is present on the outer surface area of cancer cells, which allows BXQ-350 to selectively target tumor cells and only tumor cells; this contributes to the strong safety and efficacy profile in clinical trials.
Elevation of ceramide programmed cell Death
Ceramide is pro-apoptotic. Its down regulated oncogenic pathways stimulate immuno-effector cells.
Saposin C (SapC) activates a number of enzymes increasing ceramide levels.
The elevation of ceramide creates an environment of cancer programmed cell death through several distinct mechanisms including apoptosis, necrosis, autophagy, mitophagy.
“BXQ-350’s mechanisms of action leads itself to showing the greatest potential chance of success and benefit in colorectal patients and peripheral neuropathy. Nerve damage is one of the main reasons that patients drop off chemotherapy for their colorectal cancer and BXQ-350 has been shown to possess neuroprotective properties.”
Shabnam Kazmi, M.B.A. Commercialization Advisor
Colorectal Cancer – Significant Unmet Need
Cancer continues to be a major public health problem worldwide and is the second leading cause of death in the US.1 Colorectal cancer, the second leading cause of death among cancers, is associated with late-stage diagnosis and poor outcomes. Only 15% of patients are alive at the five year mark from their diagnosis.
The current standard of care, chemotherapy, inflicts devasting side effects that potentially limits both the dose and duration of treatment while adversely affecting the quality of life and patient outcomes.
With little progress achieved over the last decade in developing safe and effective treatments, It is evident that a novel approach to treating colorectal cancer is needed.
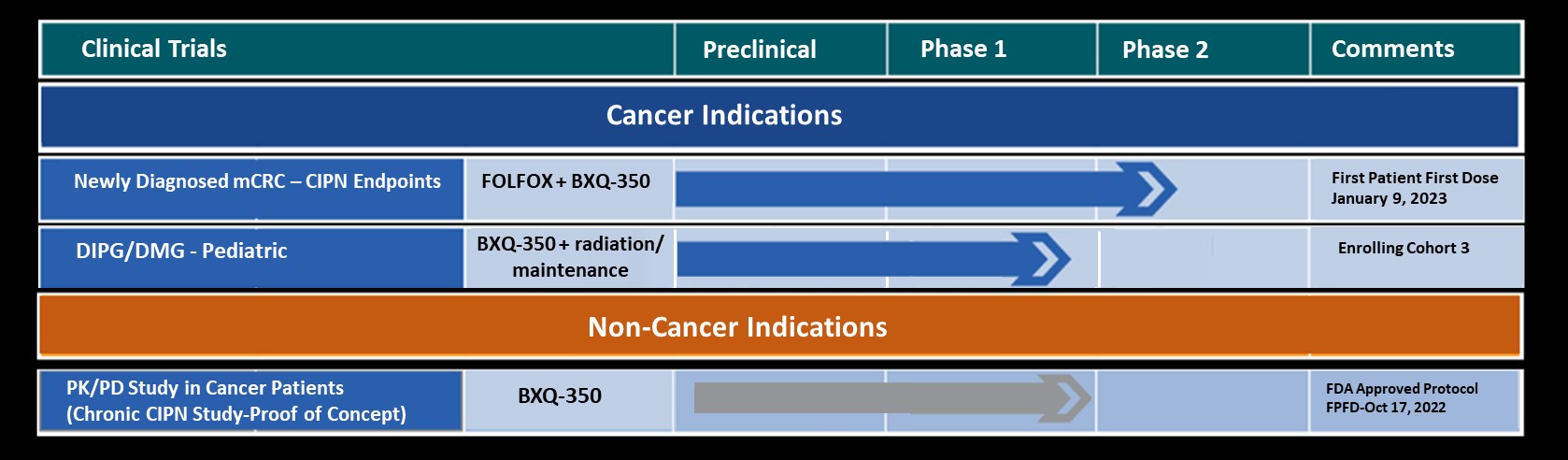
The Promise of BXQ-350 in Metastatic Colorectal Cancer (mCRC)
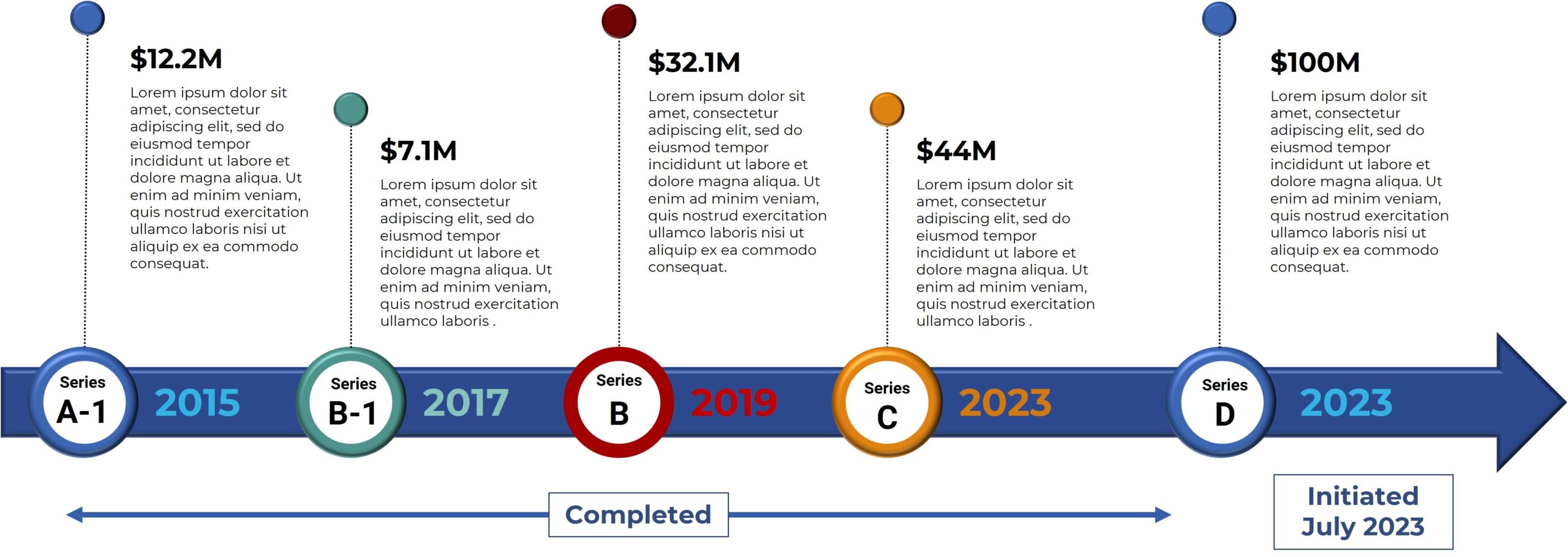
Bexion Pharmaceuticals is pursuing a robust clinical development plan to address the significant unmet needs in colorectal cancer. Early clinical results are promising
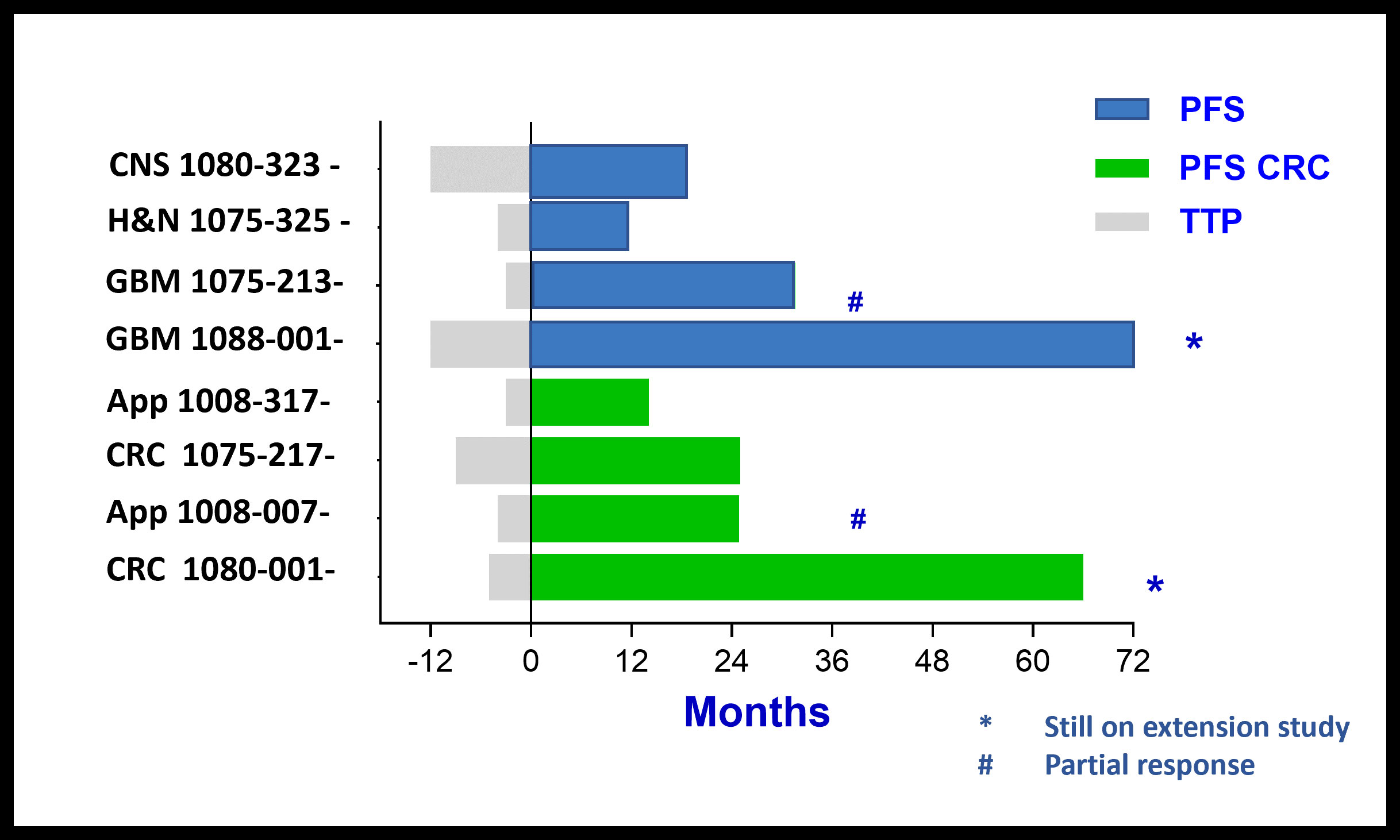
Patients with Durable Stable Disease:
PFS > 6, 12, 24 and 60+ Months
Comparison of Time To Progression (TTP) on last treatment before BXQ-350 to Progression Free Survival (PFS) for CRC patients and patients with other cancer types on BXQ-350 Study shows a positive trend for BXQ-350
PET/CT before BXQ-350
PET/CT after 18 months on BXQ-350
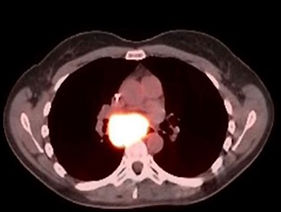
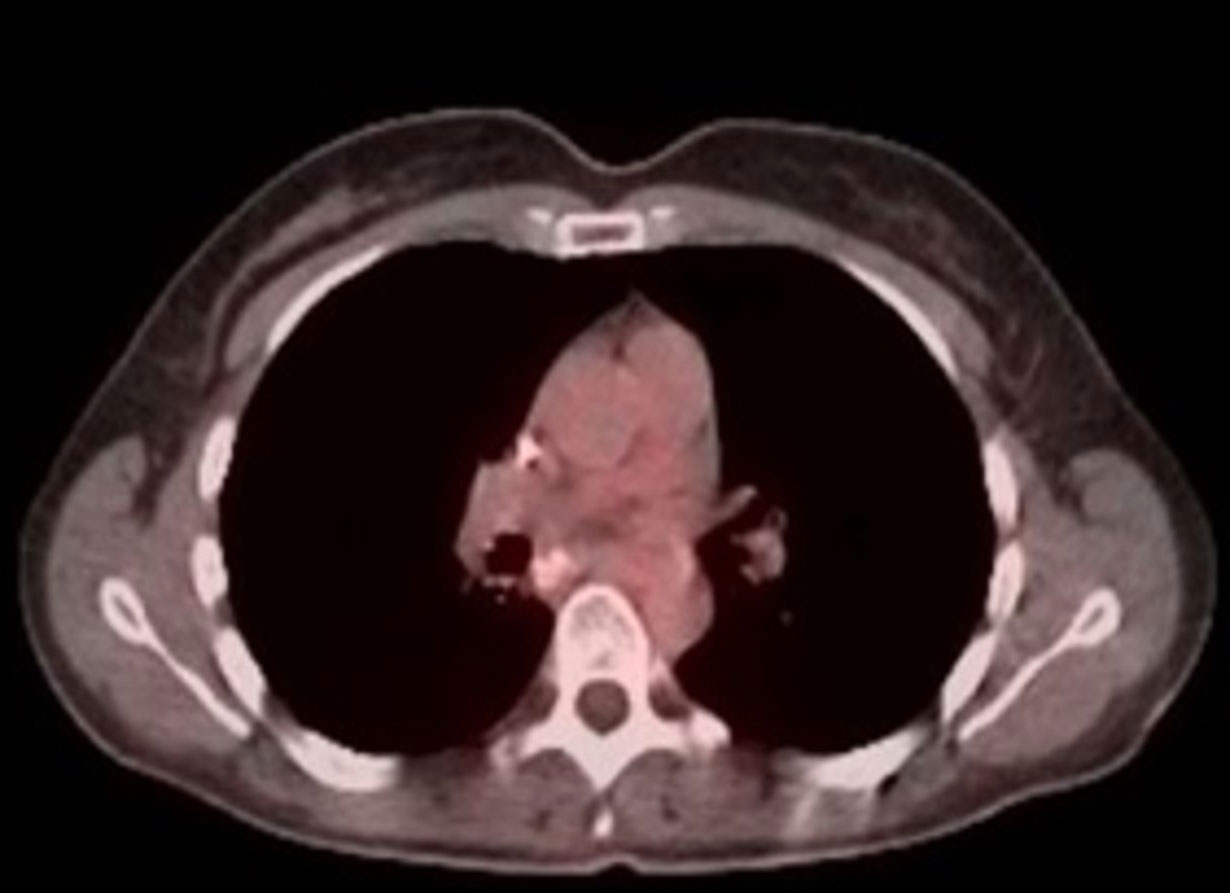
- 40-yr old female with stage 4 metastatic colorectal carcinoma
- Diagnosed in Nov 2015, previously treated with surgery, chemotherapy and radiation (>3 lines)
- Rapid progression (5 months) prior to starting BXQ-350
- Target lesion (1.5 cm) remained unchanged per Recist
- Still Stable Disease over 5 years on study
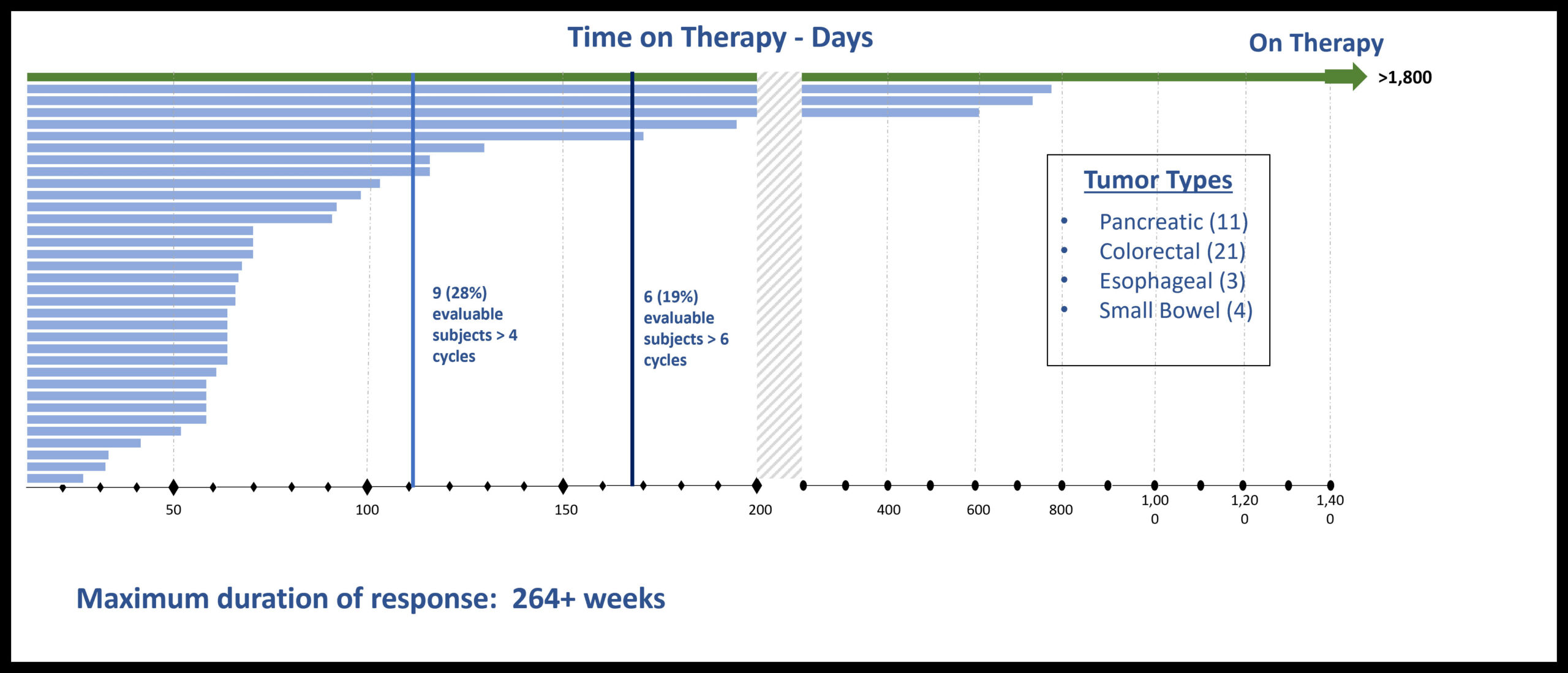
GI Cancer Subset of Phase 1 Monotherapy Study Heavily Pretreated (N-stage) Patients-Median of 7 prior therapies
What is Chemotherapy Induced Peripheral Neuropathy (CIPN)?
CIPN is a painful, debilitating condition that occurs when nerve cells of the peripheral nervous system are damaged or destroyed by chemotherapeutic agents.
CIPN is a common occurrence. Up to 90% of patients may suffer from CIPN during treatment and in nearly 50% of cases symptoms can persist 6 years after receiving chemotherapy treatment.2
CIPN Market Opportunity
In 2020, there were an estimated 3 million cases of CIPN in the United States with global annual cases surpassing 13 million.3 With cancer diagnosis and survival rates on the rise, CIPN rates are also expected to increase, warranting a heightened urgency to address this serious unmet clinical need.
“Considering the significant and growing unmet need plus the lack of effective treatments, the market potential for an FDA approved CIPN treatment would likely exceed $1 billion in annual sales.”
Jim Beach, Chief Operations Officer, Bexion Pharmaceuticals
BXQ-350 Neurotrophic Outcomes in Chemotherapy Induced Peripheral Neuropathy (CIPN) Patients
During Bexion’s Phase 1 study of BXQ-350 in adult patients with advanced solid tumors, a patient self-reported complete resolution of long term, persistent CIPN. Due to this unexpected reporting, Bexion elected to explore this finding further by reviewing data from a cohort of patients, who had entered the study with peripheral neuropathy, largely due to prior chemotherapy treatments.
Retrospective Analysis of BXQ-350 Phase 1 Data Suggests Clinical Improvement in Peripheral Neuropathy…
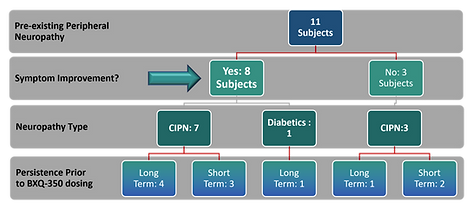
Highlights of Study4
- 8 of the 11 (73%) peripheral neuropathy sufferers reported symptom improvement (anecdotal, not primed).
- Resolution of short-term neuropathy (3 subjects) may have been coincidental to recent termination of offending chemotherapy.
- Diabetic peripheral neuropathy subject: intermittent tingling in fingers became much less frequent.
- 5 of the 6 long-term, persistent peripheral neuropathy subjects improved while on BXQ-350.
“Chemotherapy-induced peripheral neuropathy (CIPN) remains an unmet clinical need affecting far too many patients. We are excited to bring into the clinic an exciting new approach incorporating our unique knowledge at the molecular level to treating this important condition, and to potentially treat other forms of neuropathy”
Ray Takigiku, Ph.D., Founder and Chief Scientific Officer, Bexion Pharmaceuticals
Pediatric Cancer Focus
Addressing the critical needs of pediatric cancers is an important part of Bexion’s clinical development strategy.
Diffuse intrinsic pontine glioma (DIPG) and diffuse midline glioma (DMG) are extremely rare pediatric brain tumors and present a treatment dilemma due to resistance to standard therapy (radiotherapy) and aggressive clinical course. DIPG primarily affects children, with most diagnoses occurring between 5 and 7 years of age. It makes up 10-15% of all brain tumors in children, with about 150-300 new diagnoses per year in the United States. Unfortunately, fewer than 10% of children survive two years from diagnosis.
Bexion Pharmaceuticals is committed to developing therapies to address the critical need in pediatric cancers. Bexion has received Orphan Drug status under the Orphan Drug Act for its proprietary drug, BXQ-350, for the treatment of malignant glioma, including diffuse intrinsic pontine glioma (DIPG).
Additional Orphan Drug status benefits:
- Research grant eligibility for funding of Phase I and II Clinical Trials.
- Potential tax credits for certain research expenses.
- Waiver of FDA application user fees.
- Potentially faster regulatory process.
References
- National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Cancer. https://www.cdc.gov/chronicdisease/resources/publications/factsheets/cancer.htm. Accessed January 3, 2023
- Kosko, K. CIPN Experienced by Many Patients Years After Treatment. Oncology Nursing News, Cranbury NJ: https://www.oncnursingnews.com/publications/oncology-nurse/2017/september-2017/cipn-experienced-by-many-patients-years-after-treatment. Accessed January 22, 202
- The Foundation of Peripheral Neuropathy. https://www.foundationforpn.org/what-is-peripheral-neuropathy/types-risk-factors/. Accessed January 3, 2023
- Anecdotal patient reports from clinical study of BXQ-350.AA, in progress. The safety and efficacy of BXQ-350 are current being evaluated in clinical trials