Bexion Pharmaceuticals, Inc. To Present Clinical Data at 2018 ASCO Annual Meeting
FOR IMMEDIATE RELEASE Covington, KY ~ May 22,2018
Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company focused on rare brain and solid tumors, announced today that it will feature one clinical poster presentation and three online publications at the American Society of Clinical Oncology (ASCO) Annual Meeting to be held June 1-5 in Chicago, IL.
The ASCO Annual Meeting brings together more than 32,000 oncology professionals from around the world to discuss state-of-the-art treatment modalities, new therapies, and ongoing controversies in the field.
Bexion’s representation:
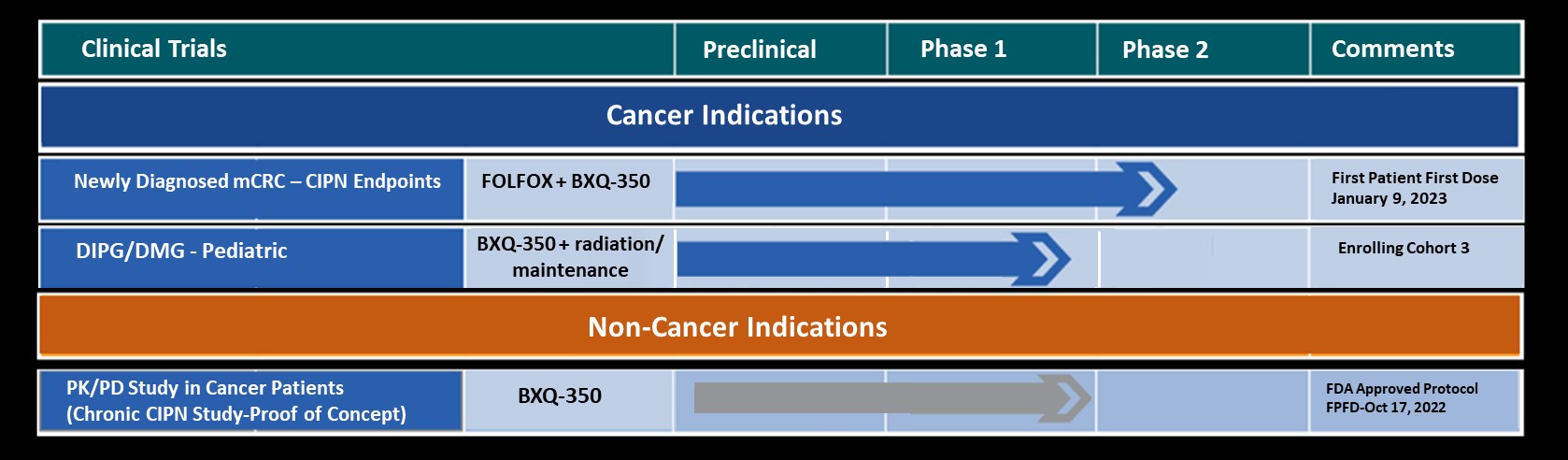
Abstract # 2517: First-in-class Phase Ia Study of BXQ-350 for Solid Tumors and Gliomas
Olivier Rixe, MD, PhD, will participate in a Q&A panel presenting data from this study during a Poster Discussion Session on Monday 4 June 2018 from 3:00-4:15 pm. Dr. Rixe’s comments will include that BXQ-350 met its Phase I Safety endpoint with no significant DLT or SAE attributed to drug at the highest planned dose. In addition, it was observed that BXQ-350 showed promising clinical activity in heavily pre-treated patients with recurrent brain cancer and advanced solid tumors.
The following three abstracts will be included online in the 2018 ASCO Annual Meeting Proceedings, a Journal of Clinical Oncology supplement, publicly released on May 16, 2018.
Absence of Indicators of Hypercoagulability and Anti-Phospholipid Syndrome in BXQ-350 First in Human Study
Therapeutic BXQ-350 nanovesicles, comprised of a lysosomal activator protein and a phosphor- lipid, did not cause anti-phospholipid syndrome (APS) or clinically significant hypercoagulability in humans.
Allometric Scaling of Preclinical Pharmacokinetic and Toxicokinetic Parameters to Predict Clinical Pharmacokinetics of BXQ-350 Saposin C Protein-Phosphatidylserine Nanovesicles
Allometric scaling of animal PK/TK data provided reasonable estimates of human PK and exposure for BXQ-350 and can be expected to have good predictive utility for extrapolating drug dose and pharmacokinetic parameters.
Combined Effect of Gemcitabine (GEM) and SapC-DOPS Nanovesicles on Pancreatic Ductal Adenocarcinoma (PDAC) in Mice
The combination of Gemcitabine and SapC-DOPS demonstrated enhanced antitumor activity in Pancreatic Ductal Adenocarcinoma (PDAC) in mice.
“These abstracts highlight the excitement we have on the potential of BXQ-350 to treat brain and multiple solid tumors; and potentially opening new avenues to treat CNS tumors” stated Dr. Ray Takigiku, Founder and CEO of Bexion.
“The University of New Mexico Comprehensive Cancer Center is pleased to take part in these Phase 1A clinical trials,” said Rixe, who is the Principal Investigator at that site and author of the Phase I protocol. “I am honored to present the preliminary results to the scientific community on Bexion’s BXQ-350 and share these exciting new developments.”
About BXQ-350
BXQ-350 is a unique formulation of a synthetically produced, human lysosomal protein, Saposin C (sphingolipid activator protein, or SapC), and the phospholipid dioleoylphosphatidylserine (DOPS).
About Bexion Pharmaceuticals
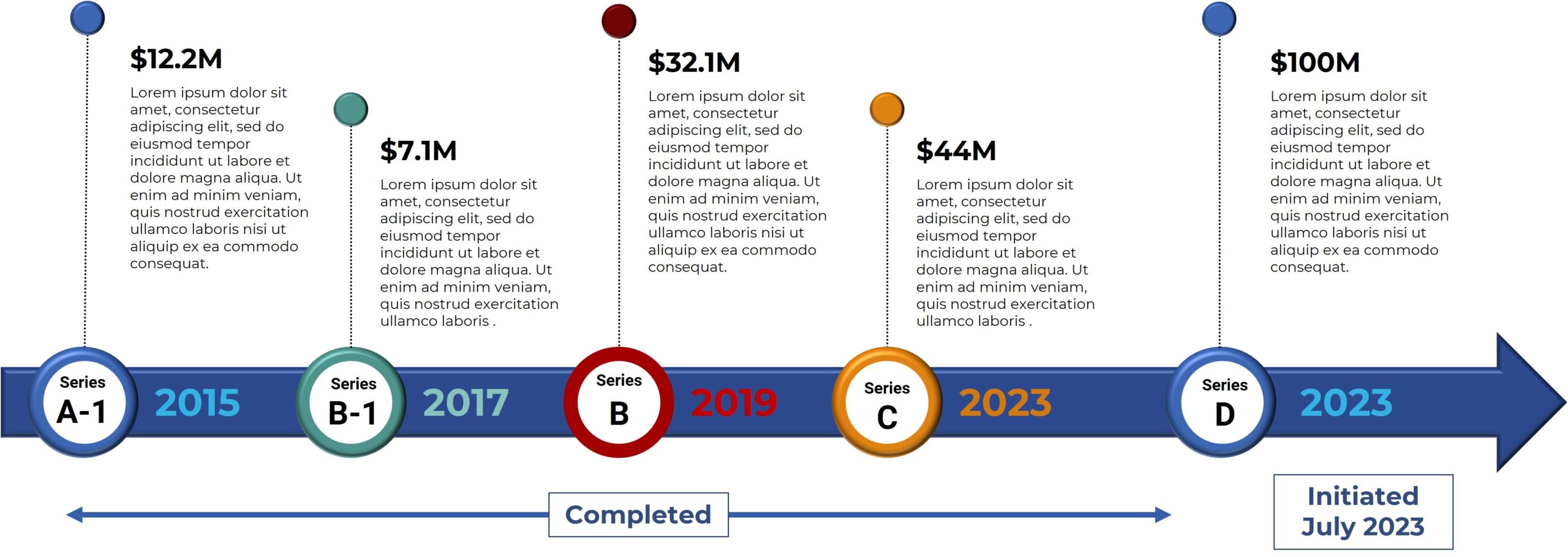
Bexion Pharmaceuticals is a privately-held biotech company focused on the development and commercialization of innovative cures for cancer. Bexion’s first-in-class biologic, BXQ-350, has demonstrated selective tumor targeting with the potential for clinical efficacy in a broad range of cancers. In 2013 the NCI awarded Bexion a prestigious “Bridge Award” of $3MM to support testing of BXQ-350 in the clinic. In February 2015, the FDA granted Bexion Orphan Drug status for Saposin C, the active ingredient in its proprietary drug BXQ-350, for the potential treatment of glioblastoma multiforme (GBM), a type of brain cancer. In June 2015, Bexion won a Tibbett’s Award by the Small Business Administration for exemplifying the very best in innovation. Currently Bexion is in a Series B Fund Raise. For more information, visit bexionpharma.dev.neptuneweb.com
About Olivier Rixe, MD, PhD
Olivier Rixe, MD, PhD, is a Professor in the Department of Internal Medicine, Division of Hematology/Oncology, at the University of New Mexico School of Medicine. He serves as the Associate Director for Clinical Research at the UNM Cancer Center. Trained as a medical oncologist, Dr. Rixe is internationally renowned for his work in Phase I clinical trials and as Principal Investigator for several neuro-oncology clinical trials. He was involved in the early development phases of many U.S. Food and Drug Administration approved agents, including oxaliplatin, camptothecins, and taxanes; and many targeted therapies including antiangiogenic compounds sunitinib, axitinib and VEGF-trap.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].