Bexion Pharmaceuticals, Inc. to Present Clinical and Pre-Clinical Data at 2020 SNO Annual Meeting
FOR IMMEDIATE RELEASE [Covington, KY: September 16, 2020]
Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company focused on the development and commercialization of innovative cures for cancer announced today it will present clinical and pre-clinical electronic posters with audio presentations at the Society of Neuro-Oncology (SNO) annual virtual conference to be held November 19-21, 2020.
“We are excited to share these latest clinical and research data on BXQ-350, adding increasing evidence to support its potential as a future treatment option for brain tumors,” said Ray Takigiku, CEO and President of Bexion Pharmaceuticals.
Clinical Poster Presentation
Abstract #: CTNI-78
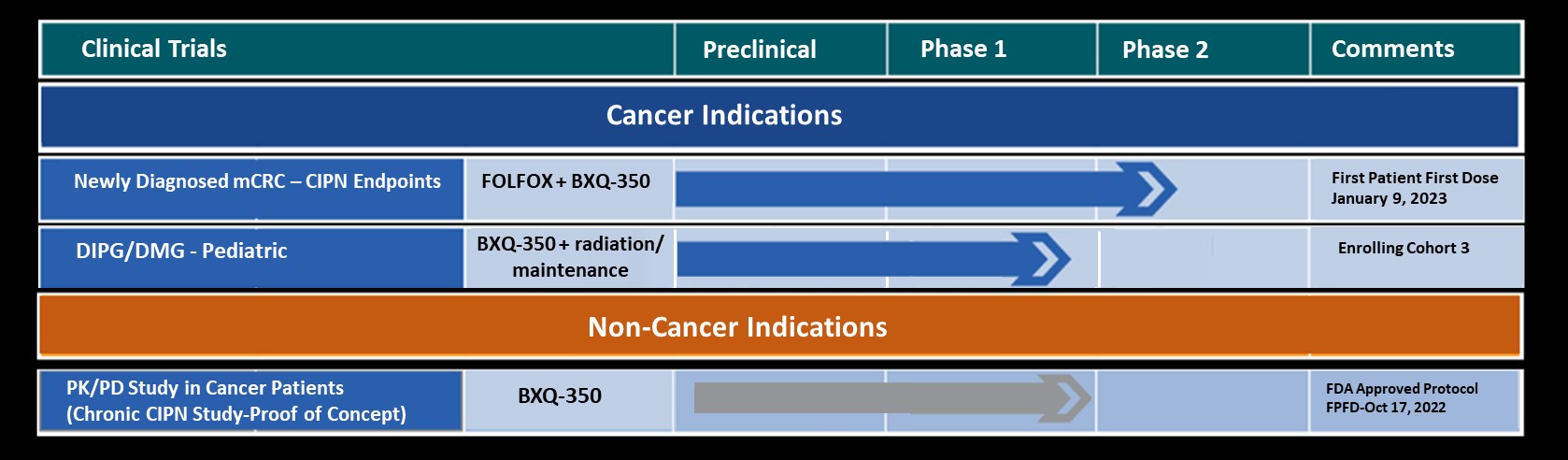
Abstract Title: A pediatric and young adult phase I dose escalation study of BXQ-350 for solid and central nervous system tumors.
Author(s): Mohamed Abdelbaki, Bhuvana Setty, Mariko D. DeWire, Timothy P. Cripe, Richard Curry
Pre-Clinical Poster Presentation
Abstract #: EXTH-49
Abstract Title: BXQ-350 to target to the lysosome and kill glioblastoma (GBM) cells via activation of apoptotic caspases in vitro.
Author(s): Laura Felix, Timothy J. Stephens, Nikhil J. Wilkins
Posters will be released November 19, 2020 at 8:00 AM EST and will be accessible on the SNO virtual meeting platform during the days of the annual meeting to SNO meeting participants.
About Bexion Pharmaceuticals
Bexion Pharmaceuticals is a clinical-stage biopharmaceutical company developing BXQ-350, a first-in-class agent composed of the multifunctional, lysosomal activator protein Saposin C and phosphatidylserine. BXQ-350 has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in brain and other solid tumors, including those that may lead to brain metastases. Bexion has completed a multi-site first-in-human Phase 1 clinical trial of BXQ-350 for solid tumors and gliomas. A Phase 1 Pediatric Trial enrollment was completed in late 2019.
Media Contact: Margaret van Gilse ●859.446-7386 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.