Bexion Pharmaceuticals Honoree at American Cancer Society Annual Ball
COVINGTON, KY. November 16, 2016 – Bexion Pharmaceuticals was awarded the “Medical Professional Award” at the American Cancer Society’s Annual Striders’ Ball held on November 5, 2016. This is the first time the honor has been bestowed on a company versus an individual medical professional.
“We are greatly honored to have been nominated, and to receive this recognition from the American Cancer Society (ACS),” stated Dr. Ray Takigiku, co-founder and CEO of Bexion. “This award has historically been given to physicians or nurses who are making significant contributions in the lives of those facing cancer. Our mission at Bexion is to save lives-transforming cancer therapies.”
Candyse Jeffries, D.M.D. and chairman of the Striders’ Ball announced, “We are so thrilled to be able to honor such an innovative and exciting company. Typically, we honor physicians that have made great strides in cancer treatment but we are so impressed with Bexion, Dr. Ray Takigiku and everything this company is doing to fight cancer that we decided to expand our award for this company”.
About BXQ-350
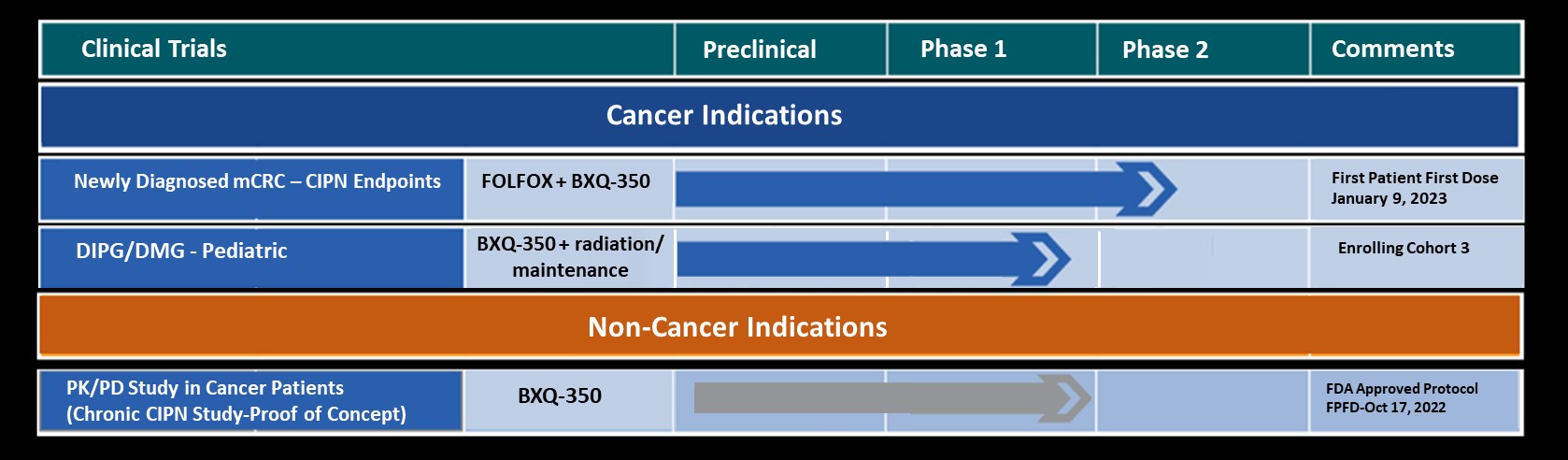
In pre-clinical animal studies, Bexion’s first-in-class biologic, BXQ-350 has been shown to induce tumor cell death in a variety of cancers. BXQ-350 is a unique formulation of a synthetically produced, human lysosomal protein. BXQ-350 is a proprietary nanovesicle formulation comprised of Saposin C (sphingolipid activator protein, or SapC) and the phospholipid dioleoylphosphatidylserine (DOPS).
About Bexion Pharmaceuticals
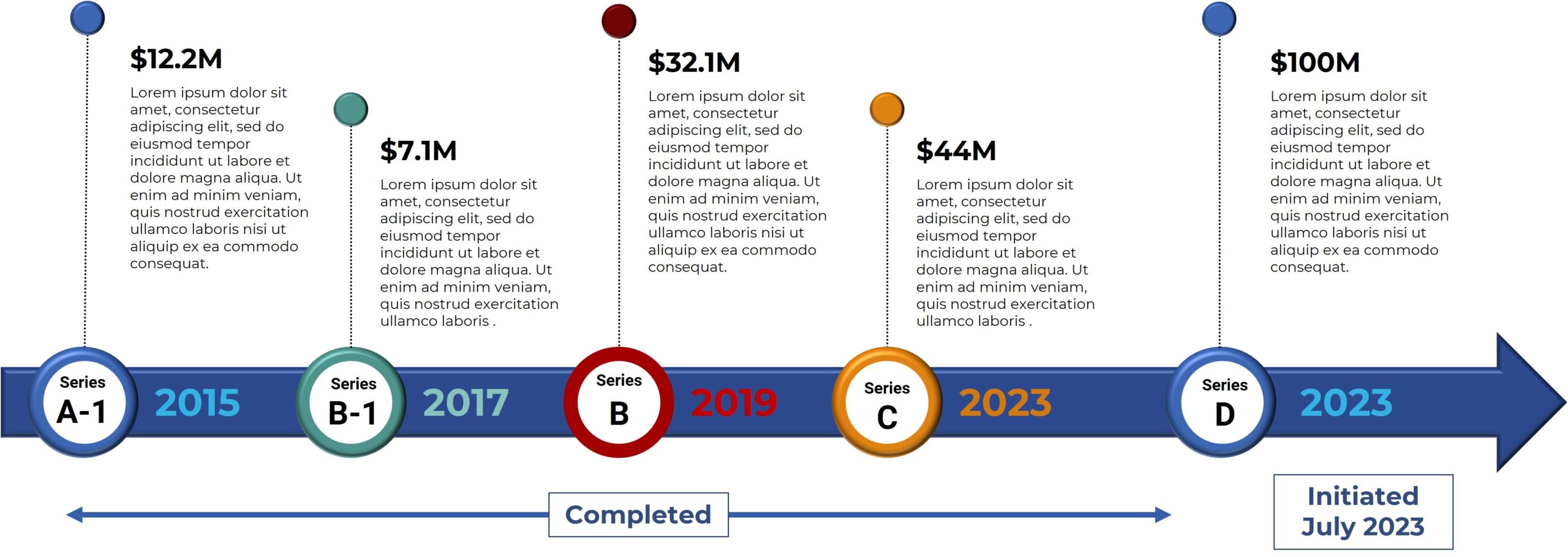
Bexion Pharmaceuticals is a privately-held biotech company focused on the development and commercialization of innovative cures for cancer. Bexion’s first-in-class biologic, BXQ-350, has demonstrated selective tumor targeting with the potential for clinical efficacy in a broad range of cancers. In 2013, the National Cancer Institute awarded Bexion a prestigious “Bridge Award” of $3M to support testing of BXQ-350 in the clinic. In February 2015, the FDA granted Bexion Orphan Drug status for Saposin C, the active ingredient in its proprietary drug BXQ-350, for the potential treatment of glioblastoma multiforme (GBM). In June 2015, Bexion won a Tibbett’s Award by the Small Business Administration for exemplifying the very best in innovation. In July 2016 the FDA cleared Bexion to initiate Phase I Clinical Trials.
About The American Cancer Society
The American Cancer Society is a global grassroots force of 2 million volunteers saving lives in every community. As the largest voluntary health organization, the Society’s efforts have contributed to a 23 percent decline in cancer death rates in the U.S. since 1991, and a 50 percent drop in smoking rates. We’re finding cures as the nation’s largest private, not-for-profit investor in cancer research, ensuring people facing cancer have the help they need and continuing the fight for access to quality health care, lifesaving screenings and more. For more information, to get help, or to join the fight, call us anytime, day or night, at (800) 227-2345 or visit cancer.org.
For more information, visit bexionpharma.dev.neptuneweb.com or contact Margaret van Gilse at [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.