Bexion Pharmaceuticals Doses First Patient in Phase I Trial of BXQ-350 For Patients with Advanced Solid Tumors at the University of Cincinnati Cancer Institute
FOR IMMEDIATE RELEASE
Margaret van Gilse
859-757-1652
[email protected]
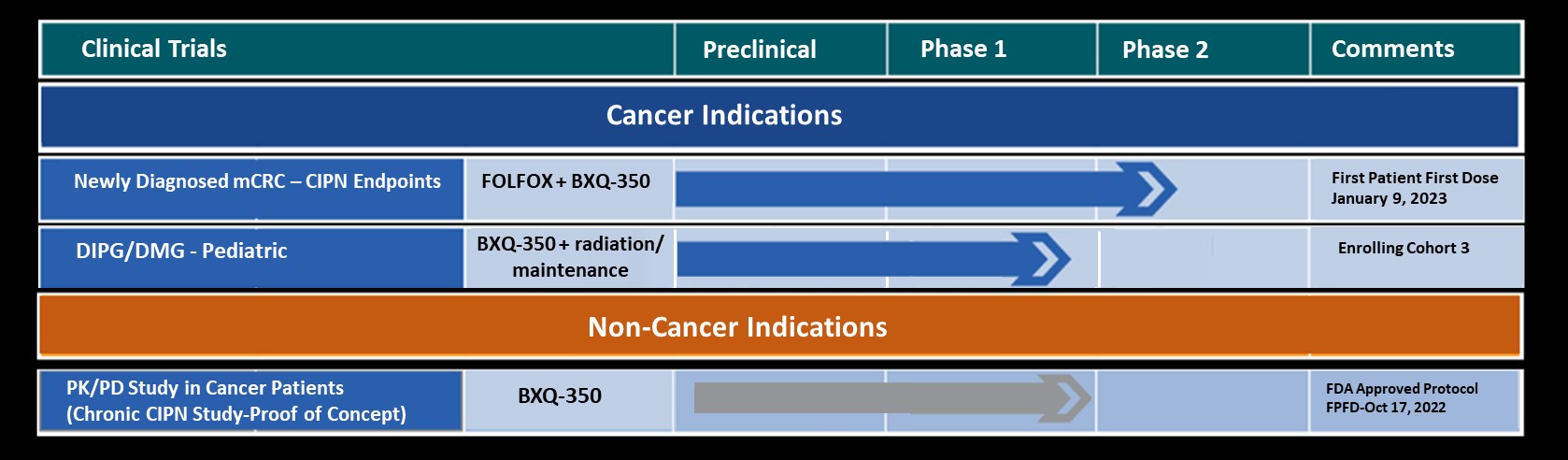
COVINGTON, KY. September 20, 2016- Bexion Pharmaceuticals, LLC (‘Bexion”) and the University of Cincinnati Cancer Institute (UCCI) announced today the dosing of the first patient in the Phase I trial of BXQ-350, a novel anti-cancer therapeutic agent.
This open-label trial will include adult patients with advanced solid tumors. The trial is designed to determine the maximum tolerated dose of BXQ-350 and to characterize its safety and pharmacokinetics. In pre-clinical animal studies, BXQ-350 was shown to induce tumor cell death in a variety of cancers, while leaving healthy cells unharmed.
“Dosing of our first ever patient with BXQ-350 is a significant milestone for Bexion,” stated Dr. Ray Takigiku, Founder and CEO of Bexion. “This trial is designed to study the safety and tolerability of BXQ-350 in patients with advanced solid tumors including glioblastoma multiforme (GBM) and may yield further insights into the potential anti-tumor activity of BXQ-350.”
Dr. John Morris, Director of the UCCI Phase I Experimental Therapeutics Program, a faculty member in the College of Medicine and a University of Cincinnati (UC) Health physician, will be leading the trial at UCCI.
“We are very excited to be part of this groundbreaking trial which could change the way patients with solid tumors, like glioblastoma multiforme—a very aggressive type of brain cancer—are treated and possibly improve outcomes and their quality of life,” he says. “It’s also a point of pride for UC and the UCCI that Dr. Xiaoyang Qi’s original research work has led to a clinical trial that could ultimately help patients with solid tumors. This is the strength of academic medicine in our community, and we’re happy to work with Bexion to make this a reality.”
Information about this Phase I trial can be found at https://www.clinicaltrials.gov/show/NCT02859857.
About BXQ-350
In pre-clinical animal studies, Bexion’s first-in-class biologic, BXQ-350 has been shown to induce tumor cell death in a variety of cancers. BXQ-350 is a synthetically produced, human lysosomal protein comprised of Saposin C (sphingolipid activator protein, or SapC) and the phospholipid, dioleoylphosphatidylserine (DOPS), which has been produced in a unique, proprietary, nanovesicle formulation.
About Bexion Pharmaceuticals
Bexion Pharmaceuticals is a privately-held biotech company focused on the development and commercialization of innovative cures for cancer. Bexion’s first-in-class biologic, BXQ-350, has demonstrated selective tumor targeting in pre-clinical animal studies, with the potential for clinical efficacy in a broad range of cancers.
In 2013, the National Cancer Institute awarded Bexion a prestigious “Bridge Award” of $3M to support testing of BXQ-350 in the clinic. In February 2015, the FDA granted Bexion Orphan Drug status for Saposin C, the active ingredient in its proprietary drug BXQ-350, for the potential treatment of GBM. In June 2015, Bexion won a Tibbett’s Award by the Small Business Administration for exemplifying the very best in innovation.
For more information, visit bexionpharma.dev.neptuneweb.com or contact Margaret van Gilse at [email protected].
Forward-Looking Statements This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” “could” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.