Bexion Pharmaceuticals Announces the Addition of Dr. Raymond J. Tesi to Board of Directors
FOR IMMEDIATE RELEASE Covington, KY November 16, 2020
Bexion Pharmaceuticals, Inc. announced today that it has added RJ. Tesi, M.D. to its Board of Directors.
Dr. Tesi has a distinguished career as a biotech entrepreneur and an experienced surgeon. Currently, he is President, Chief Executive Officer and acting Chief Medical Officer of INmune Bio (NASDAQ: INMB). Prior to his role at INMB, he held leadership positions in several biotech companies in both the development and commercial sides of the businesses with responsibility for all phases of drug development, product positioning and launch, post-approval marketing support, while supporting investor relations and business development. Dr. Tesi has been an academic transplant surgeon since 1982, receiving his MD degree from Washington University School of Medicine, and has been a Fellow of the American College of Surgery since 1991.
“We are very excited to have Dr. Tesi join our Board of Directors,” stated Dr. Ray Takigiku, Bexion’s co-founder and CEO. “Dr. Tesi brings requisite experience and expertise in clinical drug development that will help advance Bexion’s exciting programs.”
“I am excited to join the Board of Bexion,” said Dr. Tesi. “Bexion has world class technology with applications across several therapeutic areas coupled with a dedicated team of professionals. It is a recipe for success in the competitive world of biotech.”
About Bexion Pharmaceuticals
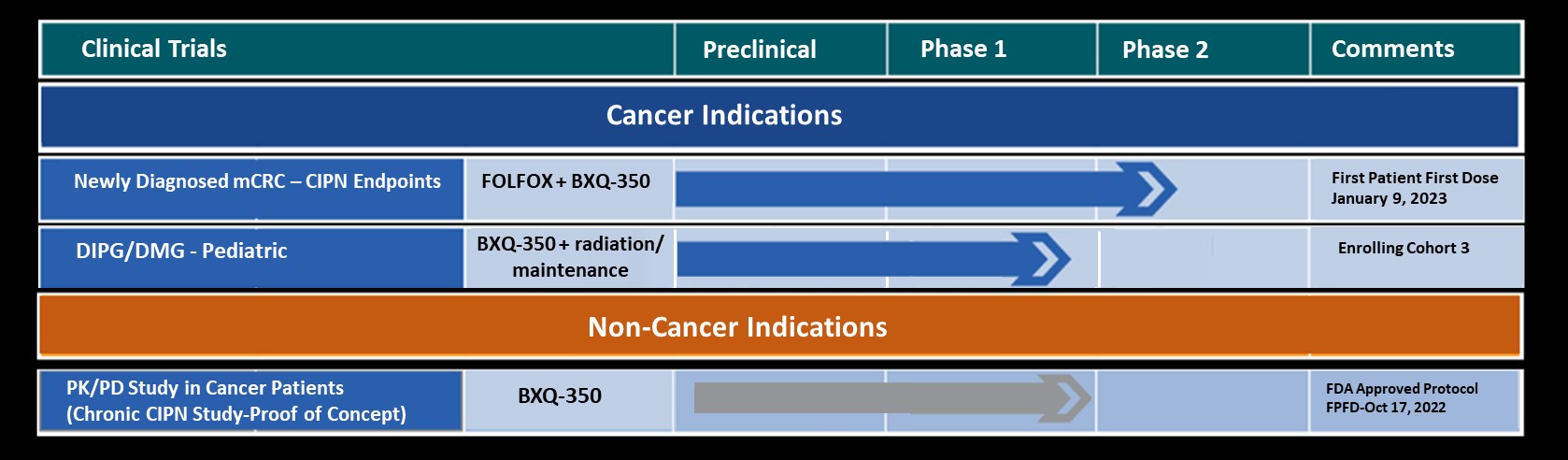
Bexion Pharmaceuticals is a clinical-stage biopharmaceutical company developing BXQ-350, a first-in-class agent composed of the multifunctional, lysosomal activator protein Saposin C and phosphatidylserine. BXQ-350 has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in brain and other solid tumors, including those that may lead to brain metastases. Bexion has completed a multi-site first-in-human Phase 1 clinical trial of BXQ-350 for solid tumors and gliomas. A Phase 1 Pediatric Trial enrollment was completed in late 2019.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.