Bexion Pharmaceuticals, Inc. Announces First Patient Dosed with BXQ-350 in the ASIST Clinical Study
Study Aims to Assess Safety and Efficacy of BXQ-350 in Combination with Standard of Care for mCRC
FOR IMMEDIATE RELEASE Covington, KY, January 10, 2022
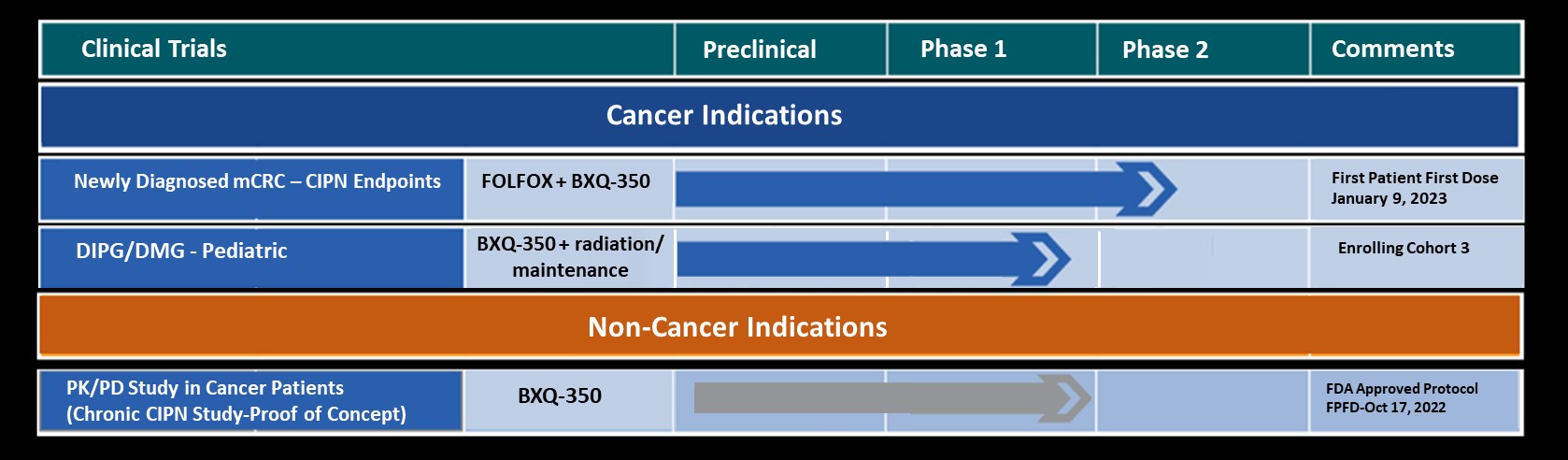
Bexion Pharmaceuticals, Inc., a clinical-stage biopharmaceutical company developing biologics for the treatment of cancer and neuropathy, announced today that the first adult patient has been dosed in the Phase 1b/2 Placebo Controlled, Double Blinded Study on the Efficacy and Safety of BXQ-350 in Combination with mFOLFOX7 and Bevacizumab in Newly Diagnosed Metastatic Colorectal Carcinoma (ASIST).
The study will assess the safety and efficacy of BXQ-350 plus modified FOLFOX7 (mFOLFOX7) and bevacizumab in participants who have newly diagnosed metastatic adenocarcinoma of the colon/rectum. The study will also evaluate if the administration of BXQ-350 with mFOLFOX7 and bevacizumab may diminish chemotherapy induced peripheral neuropathy (CIPN), enabling participants to receive the total and planned doses of mFOLFOX7.
“Dosing our first patient in this trial is a major milestone for Bexion,” stated Scott Shively, President and CEO. “BXQ-350 combined with its observed safety profile, potential efficacy, and possible neuropathy benefit makes BXQ-350 a worthwhile candidate to use in combination with standard of care treatment for mCRC to not only enhance the treatment of mCRC, but also to evaluate its ability to alleviate side effects related to CIPN.”
Initial launch of the trial includes 6 sites with expansion up to 15 sites in the United States.
Interested patients can find more information here:
https://clinicaltrials.gov/ct2/show/NCT05322590
About Bexion Pharmaceuticals
Bexion Pharmaceuticals, a clinical-stage biopharmaceutical company, is developing a new generation of biologic immunotherapy to treat solid tumor cancers and Chemotherapy Induced Peripheral Neuropathy (CIPN) with potential portfolio expansion opportunities in other cancers and broader neuropathic pain indications. Bexion’s lead drug candidate is BXQ-350, a first-in-class biologic containing the multifunctional, lysosomal activator protein, Saposin C and a phosphatidylserine.
BXQ-350, an “S1P Activator”, has demonstrated pre-clinical antitumor effects in vitro and in vivo, particularly in colorectal, brain and other solid tumors. Bexion has completed two single agent Phase 1 programs in adults and in a pediatric population. The Phase 1 programs demonstrated a strong safety profile with evidence of single agent activity across a range of tumors including Glioblastoma Multiforme (GBM), colorectal cancer and appendiceal cancer. Additionally, other clinical and non-clinical data suggest BXQ-350 has activity in chemotherapy induced peripheral neuropathy.
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
Forward-Looking Statements
This press release contains forward-looking statements, including, without limitation, statements related to Bexion Pharmaceuticals Inc.’s (the “Company”) goals, priorities, growth opportunities, new products and solutions, milestones and current and pending clinical trials. All statements contained in this presentation, other than statements of historical fact, are forward-looking statements. The words “anticipate,” “plan,” “estimate,” “expect,” “intend,” “will,” “should,” “forecast,” “project” and other similar expressions are intended to identify forward-looking statements.
These statements are based on management’s current expectations and beliefs. These expectations and beliefs are expressed in good faith and are believed to have a reasonable basis, but there can be no assurance that the statement or expectation or belief will be achieved. By their nature, forward-looking statements involve known and unknown risks, delays, uncertainties, assumptions and other factors because they relate to events and depend on circumstances that will occur in the future, whether or not outside the control of the Company. These factors include results of current or pending clinical trials, risks associated with intellectual property protection, actions by the FDA/HPB/MHRA and other factors. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. In light of these risks, uncertainties and assumptions, the future events and trends discussed in this presentation may not occur and actual results could differ materially and adversely from those anticipated, expressed or implied in the forward-looking statements.
You should not place undue reliance on forward-looking statements. The Company does not undertake an obligation to update the forward-looking statements, except as required by applicable law.