Bexion Pharmaceuticals and CTI Clinical Trial and Consulting Services Announce Collaboration on First-in-Human Trial Using BXQ-350 for the Treatment of Cancer
FOR IMMEDIATE RELEASE [Cincinnati, OH ~ September 8, 2016]
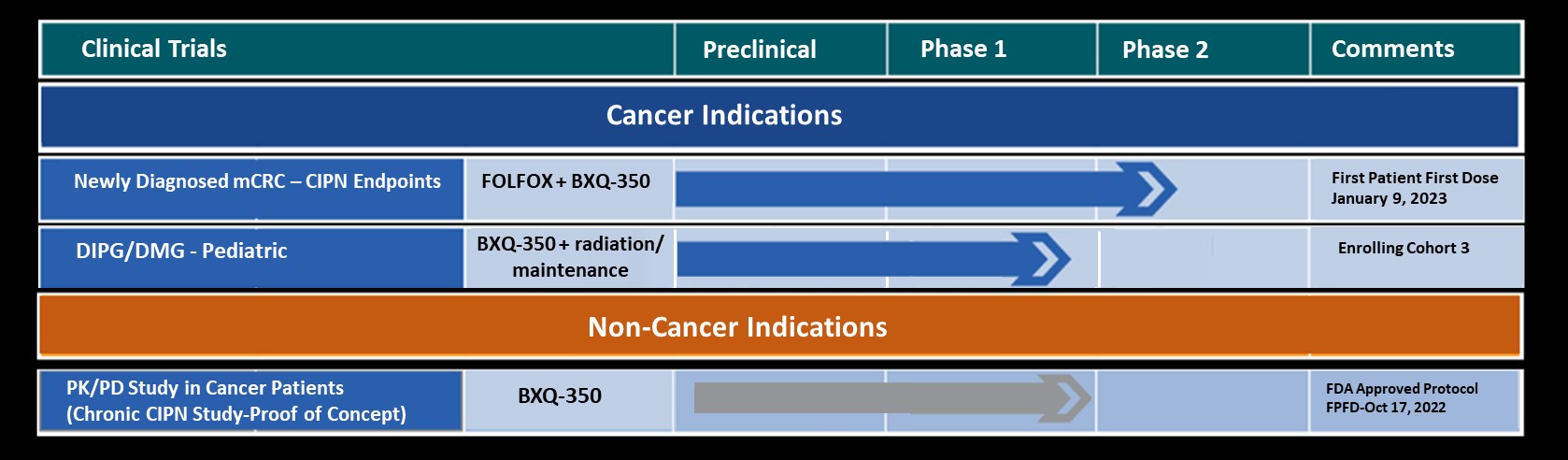
Bexion Pharmaceuticals (Bexion) and CTI Clinical Trial and Consulting Services (CTI) announce their collaboration on the First-in-Human Phase I clinical trial with BXQ-350 for the treatment of cancer. The FDA recently cleared Bexion’s application to initiate the open-label trial that will include adult patients with advanced solid tumors (including glioma, a type of brain cancer). The trial is designed to determine the maximum tolerated dose of BXQ-350 and to characterize its safety and pharmacokinetics. In pre-clinical animal studies, BXQ-350 was shown to induce tumor cell death in a variety of cancers, while leaving healthy cells unharmed.
CTI, an expert in rare disease and other life-changing therapies in critically ill patients, has been a part of several dozen First-In-Human trials over the past few years. The company recently announced the move of their headquarters to Covington, KY, where they will be in close proximity to Bexion’s corporate headquarters.
We are really enthused to be partnering with Bexion Pharmaceuticals,” according to Timothy Schroeder, CTI Founder and CEO. “They are an innovative organization with very strong regional ties – the drug was initially developed and licensed from Cincinnati Children’s Hospital Medical Center, early funding has predominantly come from the region, and the management and board have strong local connections. This program has the potential to be a game changer in the cancer arena.”
“We are very pleased to announce our partnership with CTI”, stated Dr. Ray Takigiku, Founder and CEO of Bexion. “The combination of CTI’s global expertise and outstanding customer service are critical to progressing BXQ-350 into human trials, and helping us to manage them at each of our chosen trial sites. We’re also delighted to have them as neighbors, enabling us to work more effectively together.”
Schroeder and Dr. Takigiku presented as part of the Kentucky Life Sciences Council’s Derby Partnering Summit back in May.
About BXQ-350
In pre-clinical animal studies, Bexion’s first-in-class biologic, BXQ-350 has been shown to induce tumor cell death in a variety of cancers. BXQ-350 is a unique formulation of a synthetically produced, human lysosomal protein. BXQ-350 is a proprietary nanovesicle formulation comprised of Saposin C (sphingolipid activator protein, or SapC) and the phospholipid dioleoylphosphatidylserine (DOPS).
About Bexion Pharmaceuticals
Bexion Pharmaceuticals is a privately-held biotech company focused on the development and commercialization of innovative cures for cancer. Bexion’s first-in-class biologic, BXQ-350, has demonstrated selective tumor targeting with the potential for clinical efficacy in a broad range of cancers. In 2013 the NCI awarded Bexion a prestigious “Bridge Award” of $3MM to support testing of BXQ-350 in the clinic. In February 2015, the FDA granted Bexion Orphan Drug status for Saposin C, the active ingredient in its proprietary drug BXQ-350 for the potential treatment of glioblastoma multiforme (GBM), a type of brain cancer. In June 2015, Bexion won a Tibbett’s Award by the Small Business Administration for exemplifying the very best in innovation. For more information, visit bexionpharma.dev.neptuneweb.com
Media Contact: Margaret van Gilse ●859.757.1652 ● [email protected].
About CTI Clinical Trial and Consulting Services
CTI Clinical Trial and Consulting Services is a global, privately held, full-service contract research organization (CRO), delivering a complete spectrum of clinical trial and consulting services throughout the lifecycle of development, from concept to commercialization. CTI’s focused therapeutic approach provides pharmaceutical, biotechnology, and medical device firms with clinical and disease area expertise in rare diseases, regenerative medicine/gene therapy, immunology, transplantation, nephrology, hematology/oncology, neurology, infectious diseases, hematology, cardiopulmonary, and pediatric populations. CTI also offers a fully integrated multi-specialty clinical research site that conducts phase I-IV trials. CTI has a passion for helping life-changing therapies succeed in chronically and critically ill patient populations. With clinical trial experience across 6 continents, CTI partners with research sites, patients, and sponsors to fulfill unmet medical needs. CTI is headquartered in Cincinnati, OH, with operations across North America, Europe, Latin America, and Asia-Pacific. For more information visit www.ctifacts.com
Media Contact: Allison Schroeder ● 513.598.9290 ● [email protected]
Forward-Looking Statements
This press release contains forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 that involve risks, uncertainties and assumptions that could cause Bexion’s actual results and experience to differ materially from anticipated results and expectations expressed in these forward looking statements. Bexion has in some cases identified forward-looking statements by using words such as “anticipates,” “believes,” “hopes,” “estimates,” “looks,” “expects,” “plans,” “intends,” “goal,” “potential,” “may,” “suggest,” and similar expressions. Among other factors that could cause actual results to differ materially from those expressed in forward-looking statements are Bexion’s need for, and the availability of, substantial capital in the future to fund its operations and research and development; the fact that Bexion’s compounds may not successfully complete pre-clinical or clinical testing, or be granted regulatory approval to be sold and marketed in the United States or elsewhere. You should not place undue reliance on any forward-looking statements. Bexion undertakes no obligation to release publicly the results of any revisions to any such forward-looking statements that may be made to reflect events or circumstances after the date of this press release or to reflect the occurrence of unanticipated events, except as required by applicable law or regulation.