Identifying and Developing Breakthrough Medicines
Bexion Pharmaceuticals is a mid-stage clinical company developing life-changing treatments in Oncology and CNS.
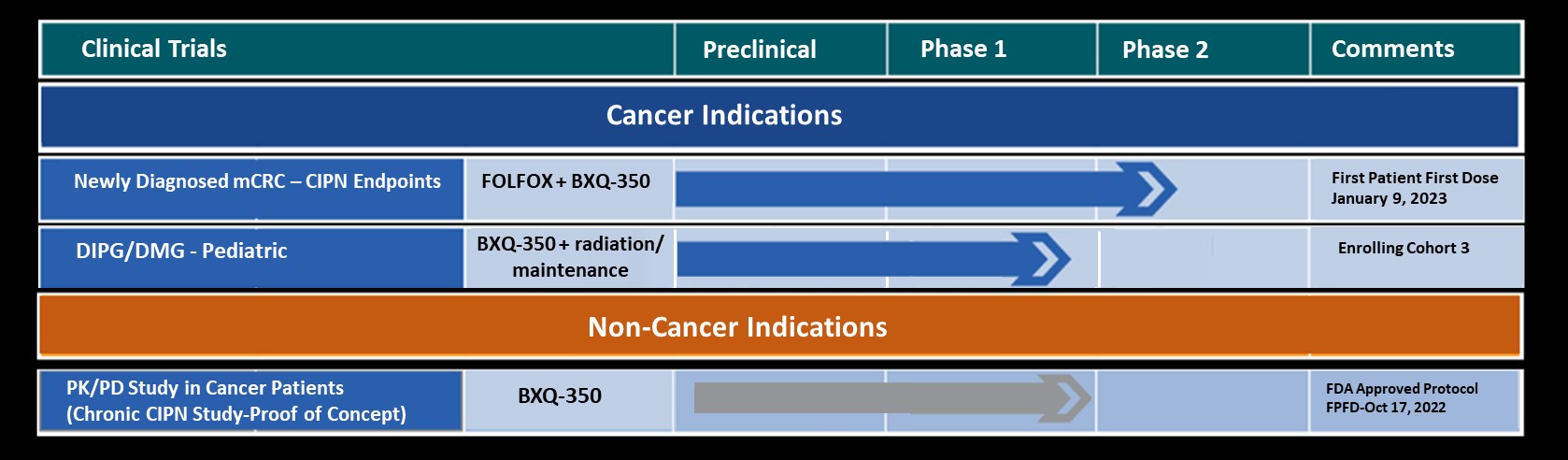
The company’s lead compound, BXQ-350, is a novel S1P modulator. With our highly experienced biotech leadership team and expertise in Oncology, we are urgently progressing our pipeline to develop life-changing oncology therapies on our path to becoming a leading biotech/pharmaceutical company.
Bexion’s Leadership

Scott Shively
President & CEO

Ray Takigiku, Ph.D.
Founder and Chief Scientific Officer

Joyce LaViscount
Chief Financial Officer

Jim Beach
Chief Operations Officer

Michael Gazda, Ph.D.
Vice President of Chemistry, Manufacturing, and Controls

Gilles Tapolsky, PhD, M.B.A.
Vice President of Pharmacology

Christy Rothwell, Ph.D., J.D.
IP, Legal Counsel

Richard C. Curry III, M.D.
Medical Affairs Advisor

Shabnam Kazmi, M.B.A.
Commercialization Advisor

Richard J. Schwen, PhD.
Regulatory Advisor
Board of Directors
Charles R. Scheper – Chairman of the Board
Mr. Chuck Scheper was the Chief Operating Officer at Great American Financial Resources, a $20 billion life and annuity company until his retirement in 2010. Prior to that position, Mr. Scheper was president and chief executive officer of Manhattan National Life Insurance Company and president of Pioneer Financial Services. Chuck is a cancer survivor and his journey to wellness led him to become active in various causes related to cancer, including Cancer Support Community, where he has served as the National Board Chairperson and board member. Currently, he is also the Chair of Catalytic Development Funding Corp of Northern Kentucky and Covington Economic Development Authority.
Scott Shively – President & CEO
Scott Shively is an experienced Founder, CEO and CCO in the US and international biopharma markets. He previously served as CEO and Co-founder of Neumentum, Inc. During his career, Mr. Shively has achieved outstanding financial results and growth in value for shareholders and has been involved in several successful exits for small-to-mid-sized biopharma companies. He spent a number of years at Pfizer where he led global commercial efforts for the Pain and CNS areas, including responsibility for products such as LYRICA® (pregabalin) and CELEBREX® (celecoxib) as well as the development pipeline.
Dr. Catherine Pearce, DHSc, MBA – Board Member
Dr. Pearce is a seasoned biopharmaceutical executive with 25 years of drug development, corporate development and C-Suite experience. Dr. Pearce led hundreds of global clinical trials at Medpace, where she later built out business development and marketing teams. At Teva Pharmaceuticals, Dr. Pearce evaluated generics for repurposing, leading to the approval of Uzedy for the treatment of schizophrenia. Dr. Pearce later co-founded CinRx, a biotech incubator, which developed 6 portfolio companies including CinCor, which was acquired by AstraZeneca in February 2023. Additionally, Dr. Pearce is the founder and CEO of JucaBio and is a member of the Board of Trustees of Xavier University and an Advisor to several start-ups. Dr. Pearce holds a BS and MBA from Xavier University, and a Doctorate of Health Sciences (DHSc) from Nova Southeastern University.
Timothy Schroeder – Board Member
Timothy Schroeder, CEO and Founder of CTI Clinical Trial and Consulting Services, has over 35 years of clinical, academic, and industry experience in global drug and device development programs. CTI, founded in 1999, is a multi-national clinical research firm with associates in North America, Europe, Latin America and Asia-Pacific. The firm, which is one of the 20 largest CROs in the world, has supported more than 100 drug and device approvals and currently works on behalf of approximately 135 global pharmaceutical and biotechnology companies.
Raymond J. Tesi, M.D.-Board Member
Dr. Tesi has a distinguished career as a biotech entrepreneur and an experienced surgeon. Currently, he is President, Chief Executive Officer and acting Chief Medical Officer of INmune Bio (NASDAQ: INMB). Prior to his role at INMB, he held leadership positions in several biotech companies in both the development and commercial sides of the businesses with responsibility for all phases of drug development, product positioning and launch, post-approval marketing support, while supporting investor relations and business development. Dr. Tesi has been an academic transplant surgeon since 1982, receiving his MD degree from Washington University School of Medicine, and has been a Fellow of the American College of Surgery since 1991.
Olivia F. Kirtley, CPA, CGMA – Board Member
Ms. Olivia Kirtley, a Certified Public Accountant and Chartered Global Management Accountant, is a highly experienced business consultant on strategic, risk and corporate governance issues. Her career spans tenures as a corporate executive and public accounting, including as Chief Financial Officer of an international company and senior tax manager at a predecessor firm to Ernst & Young. Ms. Kirtley is also a recognized leader of the accountancy profession in the U. S. and globally, having served as President and Chairman of the International Federation of Accountants (IFAC) and Chairman of the American Institute of Certified Public Accountants (AICPA). She has extensive public and private company board experience and is currently a director of U.S. Bancorp and Papa John’s International.
Keith A. Carlson – Board Member
Keith Carlson is Co-founder, CEO and Managing Partner of Roebling Capital Partners, a lower-middle-market private equity fund that completes controlling equity transactions. He leverages two decades of experience in buy and sell-side M&A transactions, corporate finance, strategy and capital markets. Mr. Carlson is also an acting Managing Director of M&A and Shareholder at VonLehman, a CPA and Advisory Firm.
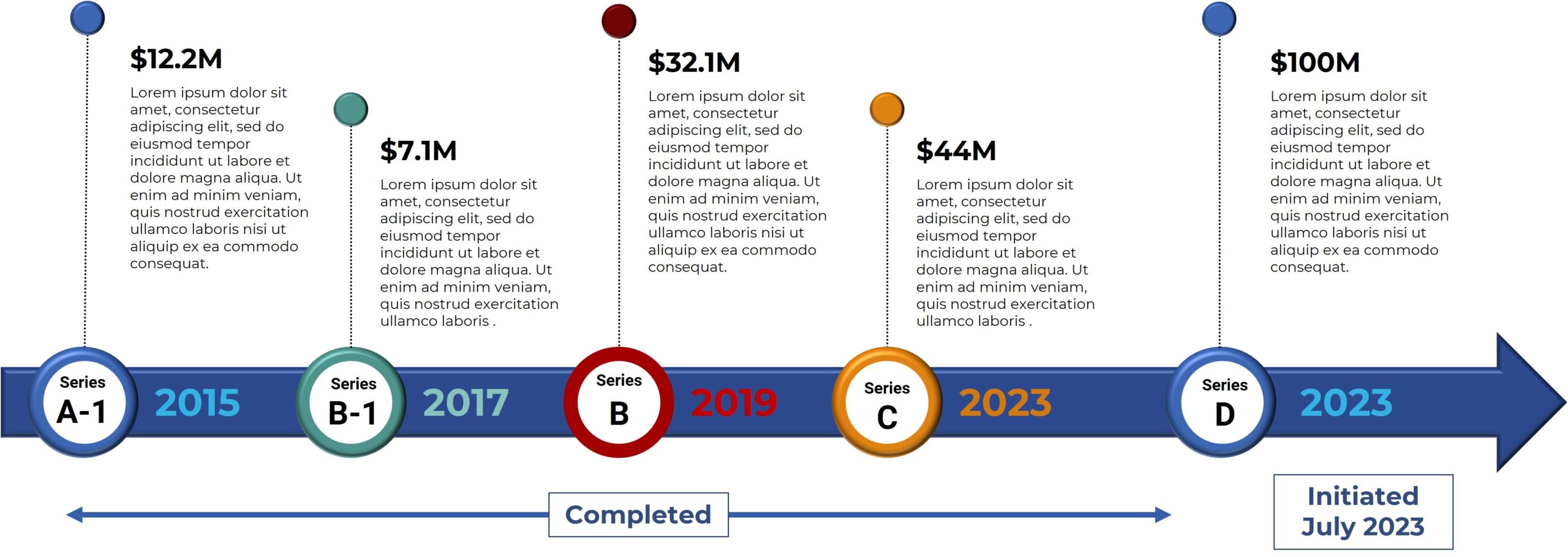
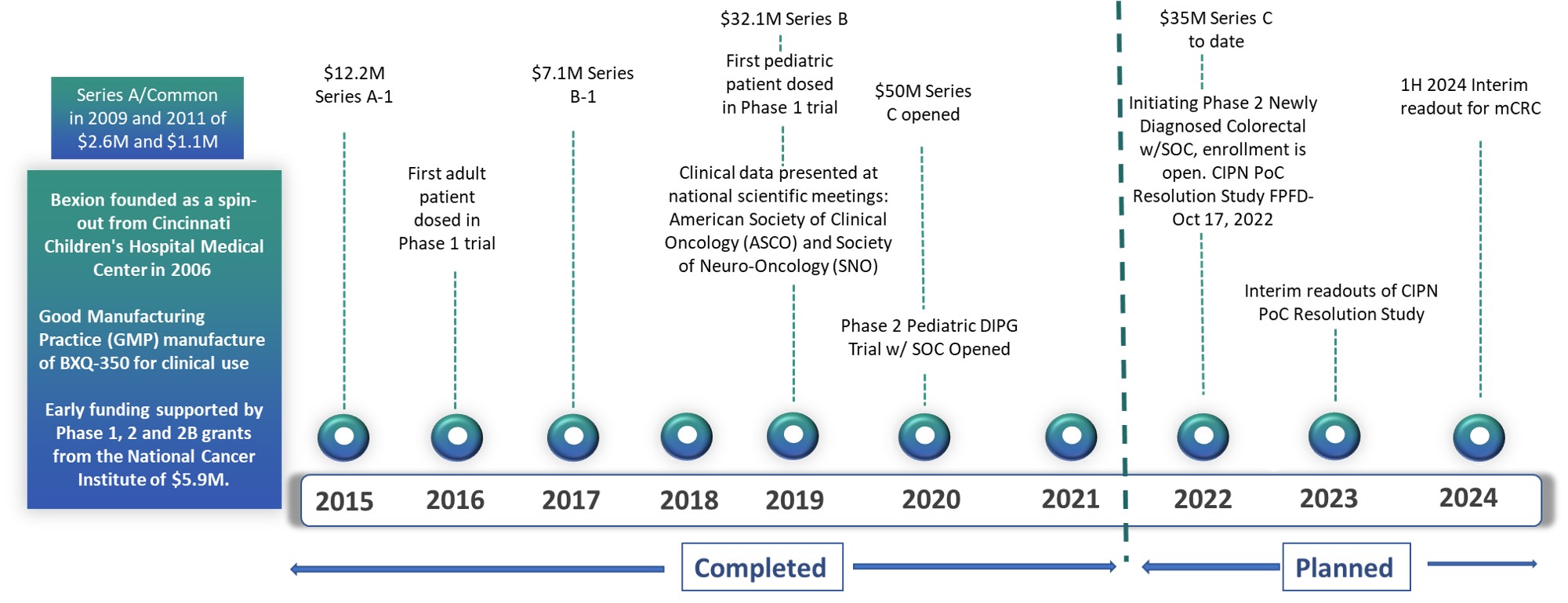
Bexion Completed and Planned Milestones
Commitment to Patient Advocacy
Bexion Pharmaceuticals is proud to collaborate with patient organizations to better support the oncology community. We actively engage with our advocacy partners to learn more about their needs which guides our ongoing activities and ensures that our priorities are best aligned with the priorities of patients and their families.
To learn more about advocacy partnership opportunities, please click on the contact information tab below.
Our Advocacy Partners